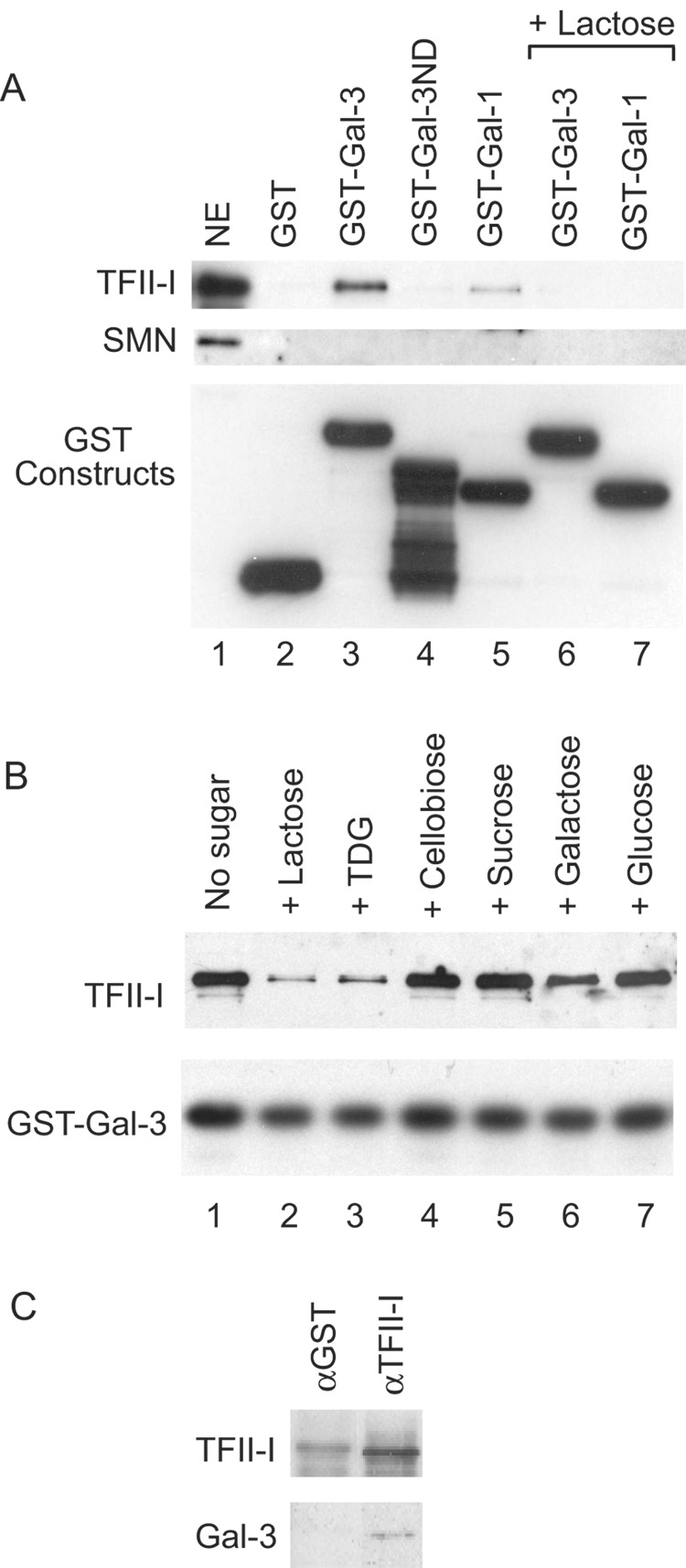
Figure 2. Immunoblotting analysis of various GST pull-down and immunopreciptation experiments.
(A) NE (lane 1), representing 15% of the material subjected to pull-down by various GST constructs: GST (lane 2), GST-Gal3 (lane 3), GST-Gal3ND (lane 4), GST-Gal1 (lane 5), GSTGal3 in the presence of 100 mM Lac (lane 6), and GST-Gal1 in the presence of 100 mM Lac (lane 7). Top panel: immunoblotting by anti-TFII-I (#558); middle panel: immunoblotting by anti-SMN; and bottom panel: immunoblotting by anti-GST to monitor the amount of GST fusion proteins bound to the glutathione beads.
(B) NE was subjected to GST-Gal3 pull-down in the absence of any carbohydrate (lane 1) or in the presence of 100 mM of Lac (lane 2), TDG (lane 3), cellobiose (lane 4), sucrose (lane 5), galactose (lane 6), and glucose (lane 7). Top panel: immunoblotting by anti-TFII-I (#558); lower panel: immunoblotting by anti-GST to monitor the amount of GST-Gal3 fusion protein bound to the glutathione beads.
(C) NE was subjected to immunoprecipitation by anti-TFII-I (#557), a rabbit antiserum affinity purified over the immunogen. Anti-GST, an antiserum that went through the same affinity purification procedure, was used as a negative control. The immunoprecipitate was subjected to blotting with anti-TFII-I and anti-Mac-2, a rat monoclonal antibody directed against Gal3.