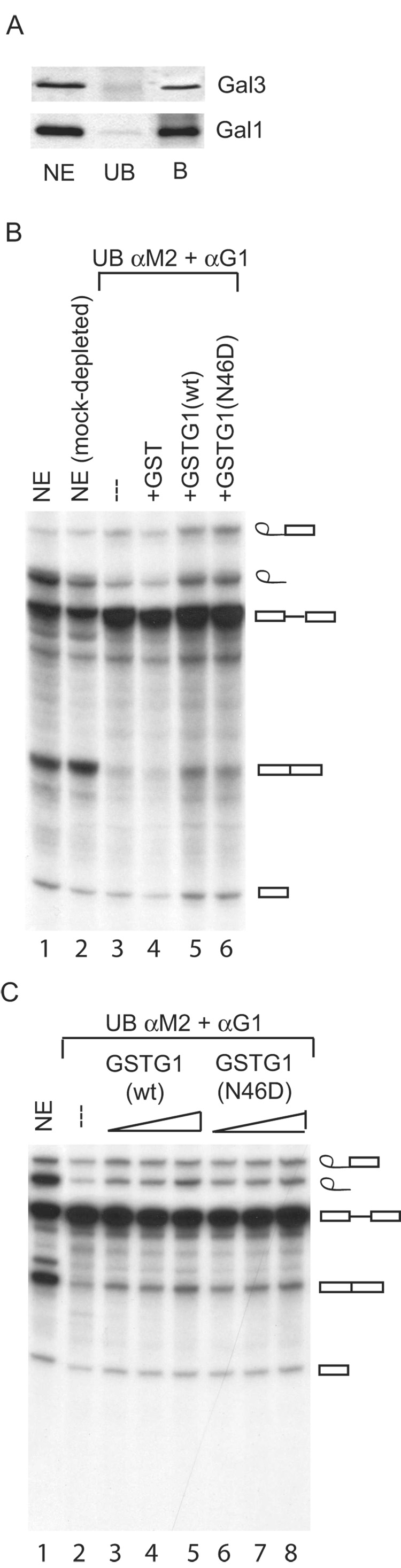
Figure 7. Comparison of the splicing activities of NE, NE after depletion of Gal1 and Gal3, and depleted NE reconstituted with GST, GST-Gal1(WT), and GST-Gal1(N46D).
NE was depleted of Gal1 and Gal3 by adsorption on beads covalently coupled with anti-Gal1 and anti-M2 (rat anti-Mac-2). Panel A: Immunoblotting for Gal1 and Gal3 in NE, the unbound (UB) fraction of the double antibody adsorption, and the bound (B) fraction. The amount of material electrophoresed in the NE and UB lanes represents ~40% of the amount electrophoresed in the B lane. Panel B: Splicing of 32P-labled MINX pre-mRNA. Lane 1, the complete (non-depleted) NE; lane 2, mock-depleted NE; lane 3, the unbound (UB) fraction of the double antibody adsorption; lanes 4–6, depleted extract reconstituted with GST, GST-Gal1(WT), and GST-Gal1(N46D), respectively. The concentration of the GST proteins was 6.5 µM. Panel C: Dose-response of the reconstitution of splicing activity by GST-Gal1(WT) and GST-Gal1(N46D). Lane 1, the complete NE; lane 2, the unbound (UB) fraction of the double antibody adsorption; lanes 3–5, reconstitution with 1 µM, 6.5 µM, and 13 µM GST-Gal1(WT); and lane 6–8, reconstitution with 1 µM, 6.5 µM, and 13 µM GST-Gal1(N46D). In panels B and C, products of the splicing reaction were analyzed by electrophoresis through a 13% polyacrylamide-urea gel and autoradiography. The positions of migration of the pre-mRNA substrate, the splicing intermediates (exon 1 and lariat-exon 2), and RNA products (ligated exon 1-exon 2 and intron lariat) are indicated on the right.