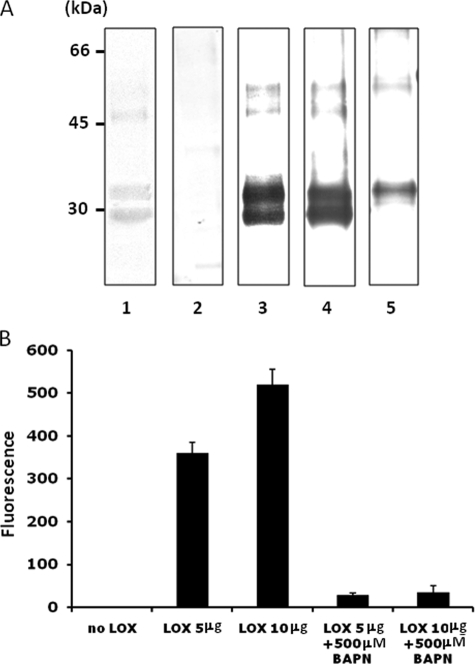
FIGURE 3.
Characterization of recombinant LOX-V5 protein. A, SDS-PAGE and WB analysis. Purified LOX-V5 protein was subjected to SDS-PAGE analysis and stained with Coomassie Brilliant Blue (lane 1) and WB analysis with NRS (lane 2), anti-(α) V5 antibody (lane 3), α LOXi antibody (Imgenex) (lane 4), and α LOXh antibody that was previously reported (27, 28) (lane 5). Two major Coomassie Brilliant Blue-stained bands were observed at ∼30- and ∼35-kDa bands. Both were immunoreactive to α V5 (lane 3) and α LOXi (lane 4) antibodies and the 35 kDa to α LOXh antibody (lane 5). Both proteins were identified as LOX by MALDI-MS (see text). The minor Coomassie Brilliant Blue-stained bands at higher molecular weight region (∼48 and ∼50 kDa) also showed immunoreactivities to those antibodies in a similar manner. No immunoreactivity was found with NRS. B, amine oxidase activity of LOX-V5 protein. Note that the amine oxidase activity of LOX-V5 protein was retained and increased in a dose-dependent manner, and the activity was nullified by 500 μm BAPN. Error bars indicate mean ± S.D. of three independent experiments.