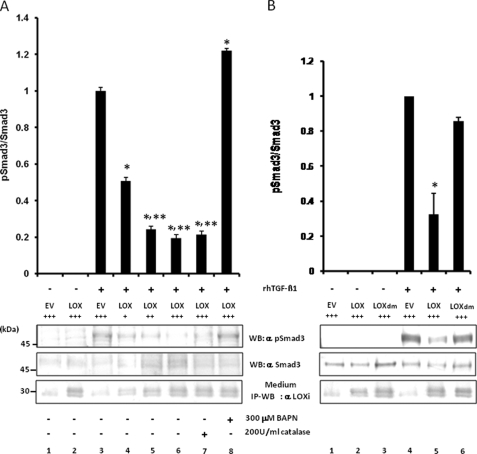
FIGURE 7.
Effect of LOX as an amine oxidase on TGF-β signaling in MC cells. A, effect of LOX overexpression and its amine oxidase activity on TGF-β signaling. TGF-β1 signaling was measured as phospho(p)-Smad3 relative to total Smad3 (pSmad3/Smad3). Without the treatment with rhTGF-β1, Smad3 phosphorylation was not induced in MC cells transfected with EV (lane 1) or those overexpressing LOX-V5 (lane 2). When rhTGF-β1(5 ng/ml) was added to EV for 30 min, Smad3 phosphorylation was induced (lane 3); however, the rhTGF-β1-induced phosphorylation level was decreased when LOX-V5 was expressed in a dose-dependent manner (lanes 4–6). The LOX inhibition of TGF-β1-induced Smad3 phosphorylation was not affected in the presence of 200 units/ml catalase (lane 7) but completely rescued in the presence of 300 μm BAPN (lane 8). Error bars indicate mean ± S.D. of three independent experiments. *, significantly different (p < 0.05) from EV. * and **, significantly different (p < 0.05) from EV and LOX+. B, effects of enzymatically active and inactive LOX on TGF-β1-induced Smad3 phosphorylation. Without TGF-β1, no phosphorylation was observed in EV (lane 1), those expressing LOX-V5 (lane 2), or LOXdm-V5 (lane 3). Smad3 phosphorylation was induced upon the treatment with rhTGF-β1 (5 ng/ml) for 30 min in EV (lane 4); however, it was significantly down-regulated by active LOX-V5 (lane 5) but almost unaffected by inactive LOXdm (lane 6). The level of LOX expression was determined by IP-WB withα LOXi antibody (lower panel). Error bars indicate mean ± S.D. of three independent experiments. *, significantly different (p < 0.05) from EV. +, ++, and +++ indicate the relative amount of EV or LOX expression vectors transfected.