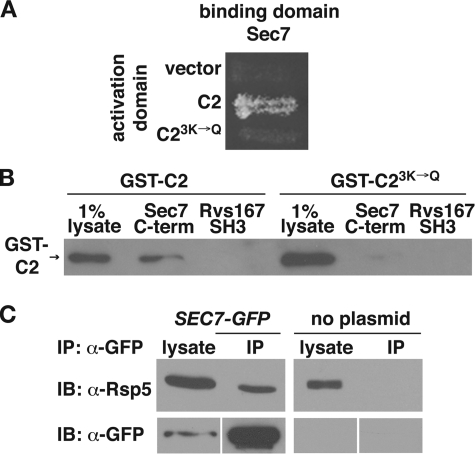
FIGURE 1.
The C2 domain of Rsp5 binds to the C terminus of Sec7. A, yeast two-hybrid analysis of the interaction between the C terminus of Sec7 (aa 1836–2009) and the wild-type or 3K→Q mutant C2 domain. Yeast strains carrying the indicated activation and binding domain plasmids were incubated on minimal medium at 30 °C. B, lysates prepared from bacteria expressing GST-C2 or GST-C23K→Q were incubated with immobilized His6-Sec71836-2009. Bound proteins were eluted by boiling, resolved by SDS-PAGE, and analyzed by immunoblotting with GST antibodies. His6-Rvs167SH3 was used as a control for nonspecific binding. The presence of equal amounts of immobilized protein in each reaction was verified by Coomassie staining of the eluted, resolved proteins (not shown). C, proteins from yeast cell lysates containing Sec7-GFP (LHY5440) and untagged Sec7 (LHY1850) were immunoprecipitated using GFP antibodies. Total lysates (0.7%, anti-GFP blot and 0.07%, anti-Rsp5 blot) and precipitated proteins were resolved by SDS-PAGE and analyzed by immunoblotting (IB) with GFP or Rsp5 antibodies. IP, immunoprecipitates.