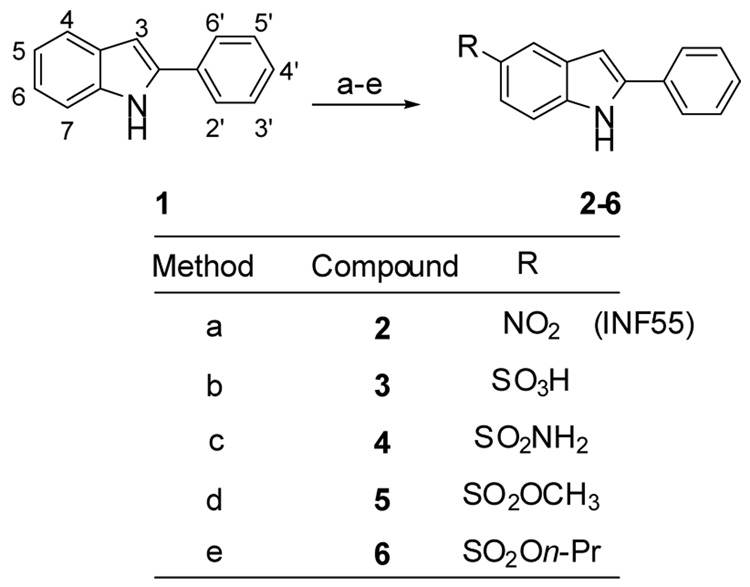
Scheme 1.
Reagents and conditions: (a) NaNO3, H2SO4 (conc.), −20 °C; 17 (b) (i) ClSO3H, 0 °C to rt, 30 mins. (ii) NaOH, H2O, rt and then 1M HCl; (c) (i) followed by (ii) NH3/THF, rt; (d) (i) followed by (ii) MeOH, pyridine, rt; (e) (i) followed by (ii) n-PrOH, pyridine rt.