Abstract
Continuous infusion of a nonlethal dose of Escherichia coli lipopolysaccharide (LPS) (0.5 mg/kg) induced early (3 h) accumulation of polymorphonuclear leukocytes (PMNL) in rat liver followed by later (30 h) greater extravasation of mononuclear phagocytes (MNP) (E. B. Rodriguez de Turco and J. A. Spitzer, J. Leukocyte Biol. 48:488-494, 1990). Nonparenchymal liver cells from rats treated for 3 and 30 h with LPS were recovered by centrifugal elutriation, yielding a 23-ml/min fraction (endothelial cells) and a 45-ml/min fraction (PMNL, Kupffer cells, and MNP), and compared for their capacity for basal and agonist-stimulated superoxide (O2-) production. Stimulation with phorbol myristate acetate and opsonized zymosan caused a dose-dependent release of O2- from the 45-ml/min fraction derived from rats treated for 3 h with saline, but not from the 23-ml/min fraction. Further purification of the 45-ml/min fraction by discontinuous density gradient centrifugation into a Kupffer and a PMNL fraction revealed that most of the agonist-induced O2- release was generated by infiltrating PMNL at this early time point of LPS infusion. By 30 h of LPS infusion, although enhancement of the phorbol-12-myristate-13-acetate- and opsonized zymosan-stimulated release of O2- was observed in the 45-ml/min fraction, but not in the 23-ml/min fraction, the maximum release of O2- was smaller than that observed in the rats treated for 3 h. Our results support the following conclusions: (i) after a 3-h LPS infusion, PMNL found in the liver in increased numbers are also highly primed for agonist-stimulated release of O2-, while Kupffer cell priming is of a lesser extent; (ii) after a 30-h infusion of LPS, infiltrating MNP found in the liver in increased numbers are primed for agonist-induced O2- release, while priming of PMNL has diminished; (iii) at both 3 and 30 h of LPS infusion, liver endothelial cells are not significantly primed for agonist-stimulated O2- release; and (iv) in vivo priming by LPS infusion at both 3 and 30 h was not reversed by the experimental method used for cell recovery (ca. 3 h), thus suggesting that in vivo LPS priming of O2- release may ultimately lead to severe impairment of liver function and metabolism observed during endotoxemia and sepsis if not therapeutically blocked at an early time point.
Full text
PDF

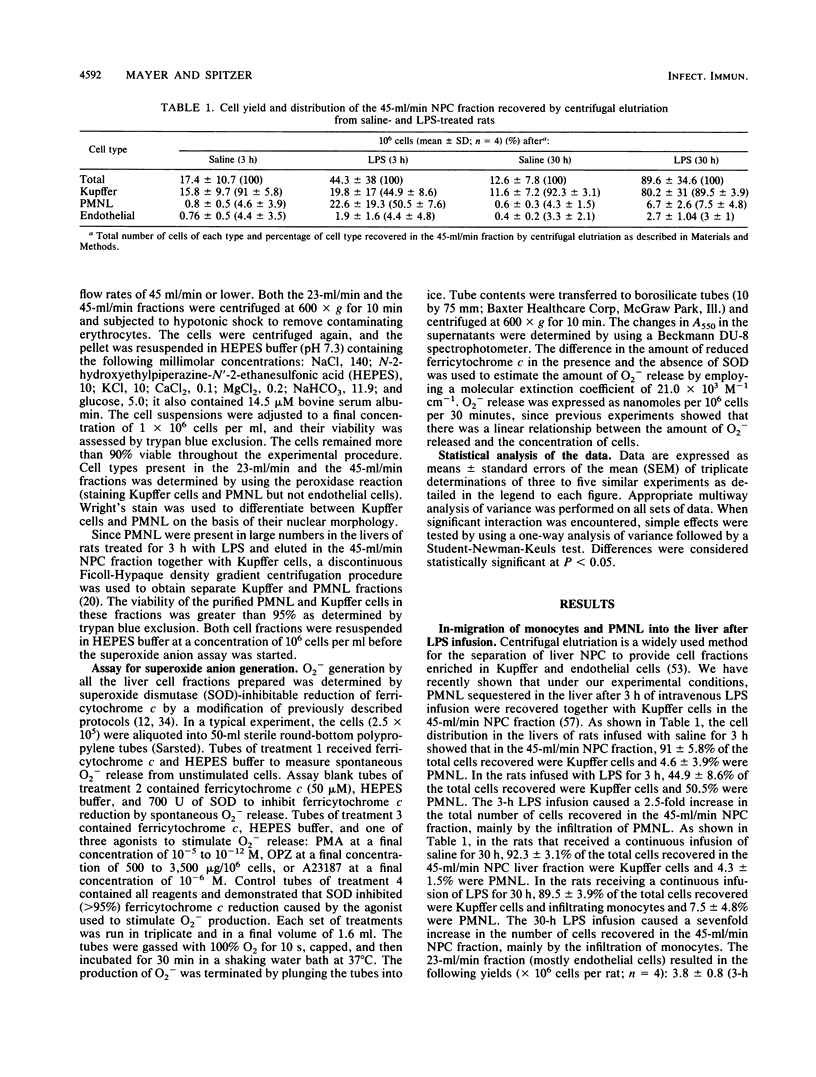
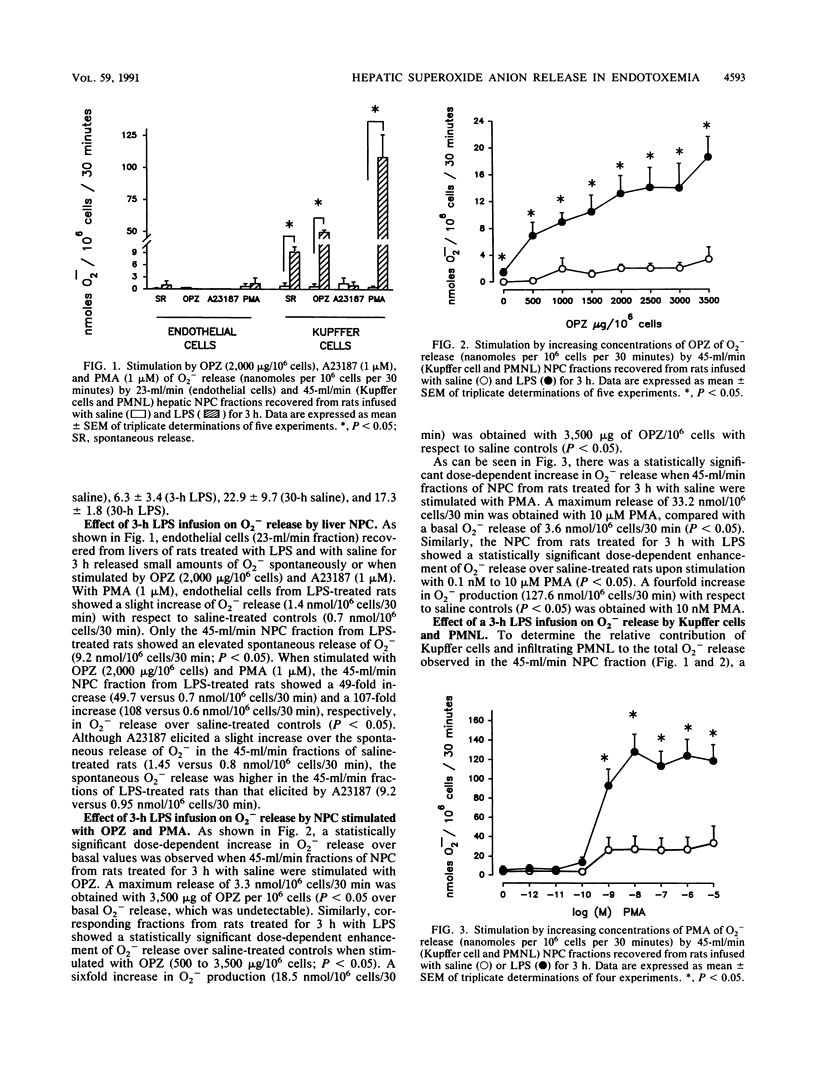
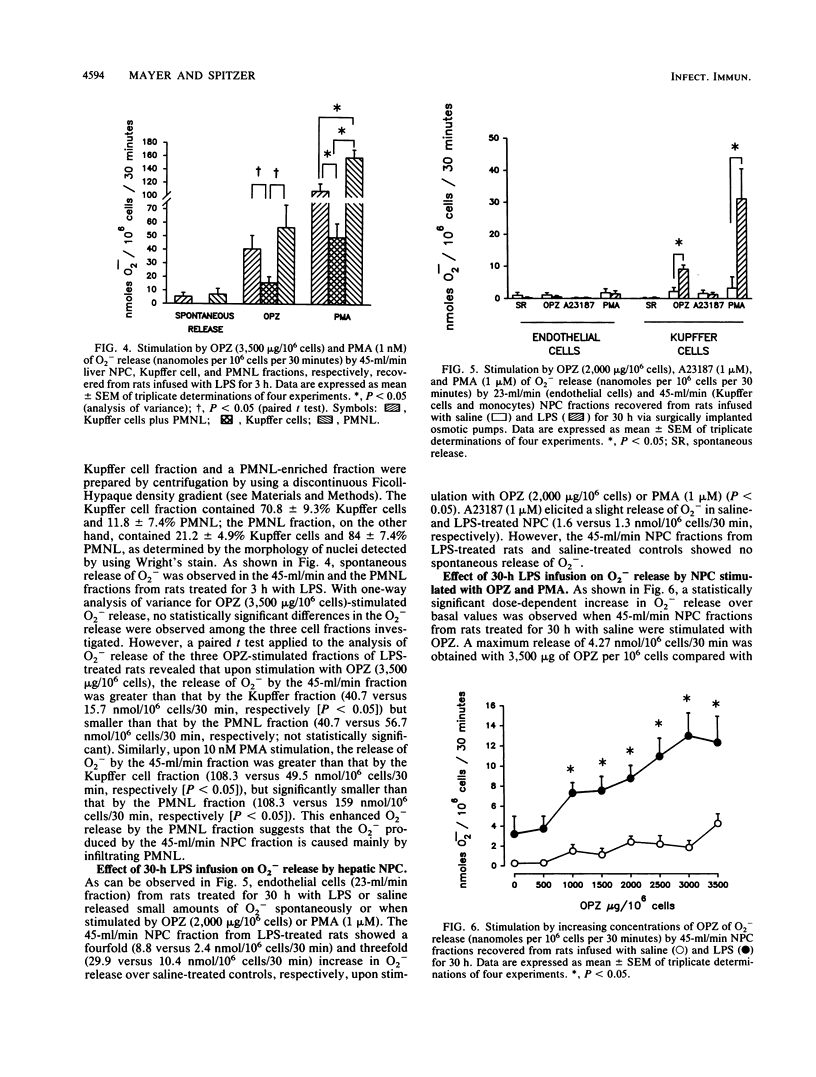
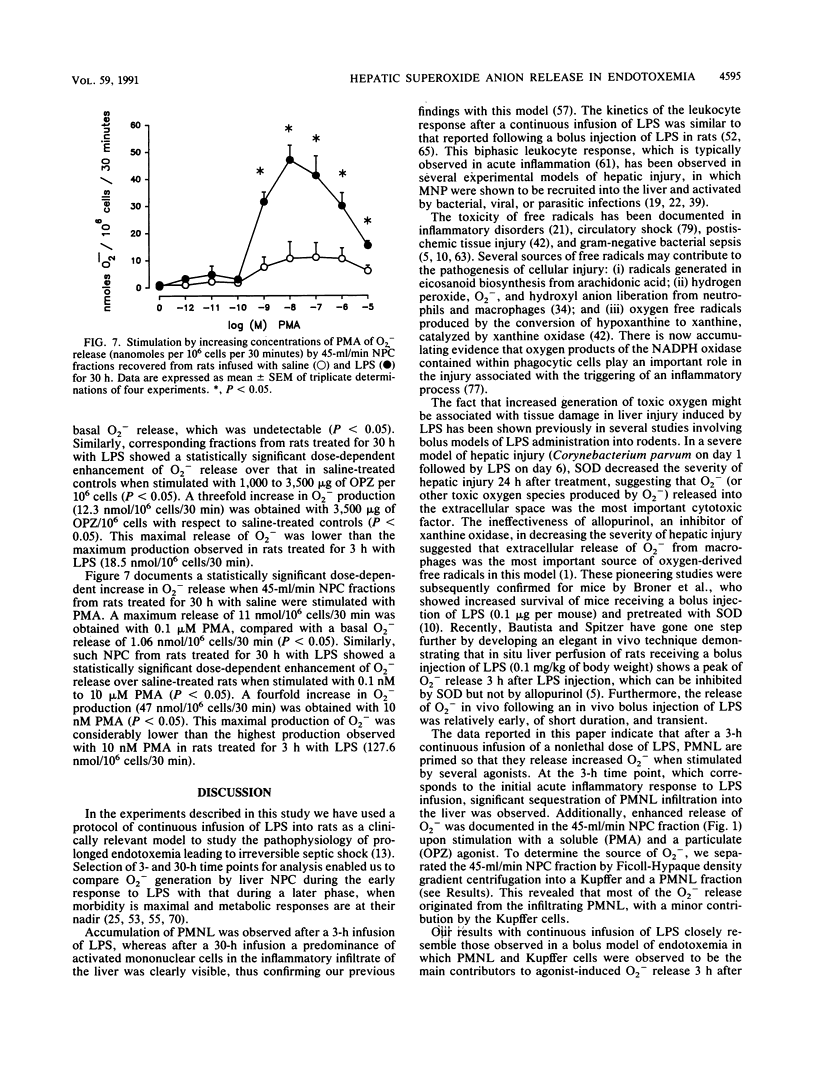



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M. J., Bentley I. S., Tanner A. R., Saunders P. K., Millward-Sadler G. H., Wright R. Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology. 1985 Nov;89(5):1114–1122. doi: 10.1016/0016-5085(85)90218-5. [DOI] [PubMed] [Google Scholar]
- Arthur M. J., Kowalski-Saunders P., Wright R. Corynebacterium parvum-elicited hepatic macrophages demonstrate enhanced respiratory burst activity compared with resident Kupffer cells in the rat. Gastroenterology. 1986 Jul;91(1):174–181. doi: 10.1016/0016-5085(86)90455-5. [DOI] [PubMed] [Google Scholar]
- BROITMAN S. A., GOTTLIEB L. S., ZAMCHECK N. INFLUENCE OF NEOMYCIN AND INGESTED ENDOTOXIN IN THE PATHOGENESIS OF CHOLINE DEFICIENCY CIRRHOSIS IN THE ADULT RAT. J Exp Med. 1964 Apr 1;119:633–642. doi: 10.1084/jem.119.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balis J. U., Rappaport E. S., Gerber L., Fareed J., Buddingh F., Messmore H. L. A primate model for prolonged endotoxin shock. Blood-vascular reactions and effects of glucocorticoid treatment. Lab Invest. 1978 Apr;38(4):511–523. [PubMed] [Google Scholar]
- Bautista A. P., Mészáros K., Bojta J., Spitzer J. J. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol. 1990 Aug;48(2):123–128. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- Bautista A. P., Spitzer J. J. Superoxide anion generation by in situ perfused rat liver: effect of in vivo endotoxin. Am J Physiol. 1990 Dec;259(6 Pt 1):G907–G912. doi: 10.1152/ajpgi.1990.259.6.G907. [DOI] [PubMed] [Google Scholar]
- Berton G., Gordon S. Modulation of macrophage mannosyl-specific receptors by cultivation on immobilized zymosan. Effects on superoxide-anion release and phagocytosis. Immunology. 1983 Aug;49(4):705–715. [PMC free article] [PubMed] [Google Scholar]
- Bouwens L., Wisse E. Proliferation, kinetics, and fate of monocytes in rat liver during a zymosan-induced inflammation. J Leukoc Biol. 1985 May;37(5):531–543. doi: 10.1002/jlb.37.5.531. [DOI] [PubMed] [Google Scholar]
- Broner C. W., Shenep J. L., Stidham G. L., Stokes D. C., Hildner W. K. Effect of scavengers of oxygen-derived free radicals on mortality in endotoxin-challenged mice. Crit Care Med. 1988 Sep;16(9):848–851. doi: 10.1097/00003246-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Camara D. S., Caruana J. A., Jr, Schwartz K. A., Montes M., Nolan J. P. D-Galactosamine liver injury: absorption of endotoxin and protective effect of small bowel and colon resection in rabbits. Proc Soc Exp Biol Med. 1983 Feb;172(2):255–259. doi: 10.3181/00379727-172-41555. [DOI] [PubMed] [Google Scholar]
- Cerasoli F., Jr, McKenna P. J., Rosolia D. L., Albertine K. H., Peters S. P., Gee M. H. Superoxide anion release from blood and bone marrow neutrophils is altered by endotoxemia. Circ Res. 1990 Jul;67(1):154–165. doi: 10.1161/01.res.67.1.154. [DOI] [PubMed] [Google Scholar]
- Danner R. L., Elin R. J., Hosseini J. M., Wesley R. A., Reilly J. M., Parillo J. E. Endotoxemia in human septic shock. Chest. 1991 Jan;99(1):169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- Deaciuc I. V., Spitzer J. A. Rat liver free cytosolic Ca2+ and glycogen phosphorylase in endotoxicosis and sepsis. Am J Physiol. 1986 Nov;251(5 Pt 2):R984–R995. doi: 10.1152/ajpregu.1986.251.5.R984. [DOI] [PubMed] [Google Scholar]
- Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur J Biochem. 1990 Sep 11;192(2):245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- Deitch E. A. Bacterial translocation of the gut flora. J Trauma. 1990 Dec;30(12 Suppl):S184–S189. doi: 10.1097/00005373-199012001-00037. [DOI] [PubMed] [Google Scholar]
- Dewald B., Payne T. G., Baggiolini M. Activation of NADPH oxidase of human neutrophils. Potentiation of chemotactic peptide by a diacylglycerol. Biochem Biophys Res Commun. 1984 Nov 30;125(1):367–373. doi: 10.1016/s0006-291x(84)80377-0. [DOI] [PubMed] [Google Scholar]
- Diesselhoff-den Dulk M. M., Crofton R. W., van Furth R. Origin and kinetics of Kupffer cells during an acute inflammatory response. Immunology. 1979 May;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Ferluga J., Allison A. C. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet. 1978 Sep 16;2(8090):610–611. doi: 10.1016/s0140-6736(78)92828-3. [DOI] [PubMed] [Google Scholar]
- Fish R. E., Burns A. H., Lang C. H., Spitzer J. A. Myocardial dysfunction in a nonlethal, nonshock model of chronic endotoxemia. Circ Shock. 1985;16(3):241–252. [PubMed] [Google Scholar]
- Fish R. E., Lang C. H., Spitzer J. A. Regional blood flow during continuous low-dose endotoxin infusion. Circ Shock. 1986;18(4):267–275. [PubMed] [Google Scholar]
- Fish R. E., Spitzer J. A. Continuous infusion of endotoxin from an osmotic pump in the conscious, unrestrained rat: a unique model of chronic endotoxemia. Circ Shock. 1984;12(2):135–149. [PubMed] [Google Scholar]
- Fox E. S., Thomas P., Broitman S. A. Hepatic mechanisms for clearance and detoxification of bacterial endotoxins. J Nutr Biochem. 1990 Dec;1(12):620–628. doi: 10.1016/0955-2863(90)90020-l. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Bøg-Hansen T. C., Back U., Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980 May;28(2):373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Freudenberg N., Galanos C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982 Feb;63(1):56–65. [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Kleine B., Galanos C. The fate of lipopolysaccharide in rats: evidence for chemical alteration in the molecule. Rev Infect Dis. 1984 Jul-Aug;6(4):483–487. doi: 10.1093/clinids/6.4.483. [DOI] [PubMed] [Google Scholar]
- GYORGY P. Antibiotics and liver injury. Ann N Y Acad Sci. 1954 May 10;57(6):925–931. doi: 10.1111/j.1749-6632.1954.tb36471.x. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Nomoto K., Matsuzaki T., Yokokura T., Mutai M. Oxygen radical production by peritoneal macrophages and Kupffer cells elicited with Lactobacillus casei. Infect Immun. 1984 Apr;44(1):61–67. doi: 10.1128/iai.44.1.61-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Measurement of O2- secreted by monocytes and macrophages. Methods Enzymol. 1984;105:365–369. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
- Kelly P. M., Heryet A. R., McGee J. O. Kupffer cell number is normal, but their lysozyme content is reduced in alcoholic liver disease. J Hepatol. 1989 Mar;8(2):173–180. doi: 10.1016/0168-8278(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- LUCKEY T. D., REYNIERS J. A., GYORGY P., FORBES M. Germfree animals and liver necrosis. Ann N Y Acad Sci. 1954 May 10;57(6):932–935. doi: 10.1111/j.1749-6632.1954.tb36472.x. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- MacDonald J. R., Beckstead J. H., Smuckler E. A. An ultrastructural and histochemical study of the prominent inflammatory response in D(+)-galactosamine hepatotoxicity. Br J Exp Pathol. 1987 Apr;68(2):189–199. [PMC free article] [PubMed] [Google Scholar]
- Markert M., Andrews P. C., Babior B. M. Measurement of O2- production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984;105:358–365. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McGuire G. P., Pearl R. G. Sepsis and the trauma patient. Crit Care Clin. 1990 Jan;6(1):121–146. [PubMed] [Google Scholar]
- Mochida S., Ogata I., Ohta Y., Yamada S., Fujiwara K. In situ evaluation of the stimulatory state of hepatic macrophages based on their ability to produce superoxide anions in rats. J Pathol. 1989 May;158(1):67–71. doi: 10.1002/path.1711580113. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan J. P., Ali M. V. Endotoxin and the liver. II. Effect of tolerance on carbon tetrachloride-induced injury. J Med. 1973;4(1):28–38. [PubMed] [Google Scholar]
- Nolan J. P. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981 Sep-Oct;1(5):458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- Nolan J. P., Leibowitz A. I. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b--an antiendotoxin. Gastroenterology. 1978 Sep;75(3):445–449. [PubMed] [Google Scholar]
- Nolan J. P., Leibowitz A. I., Vladutiu A. O. Influence of carbon tetrachloride on circulating endotoxin after exogenous administration of endotoxin in rats. Proc Soc Exp Biol Med. 1980 Dec;165(3):453–456. doi: 10.3181/00379727-165-41003. [DOI] [PubMed] [Google Scholar]
- Pereira C. A., Steffan A. M., Koehren F., Douglas C. R., Kirn A. Increased susceptibility of mice to MHV 3 infection induced by hypercholesterolemic diet: impairment of Kupffer cell function. Immunobiology. 1987 May;174(3):253–265. doi: 10.1016/S0171-2985(87)80001-3. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaro A. M., Laskin D. L. Accumulation of activated mononuclear phagocytes in the liver following lipopolysaccharide treatment of rats. J Leukoc Biol. 1986 Jul;40(1):29–41. doi: 10.1002/jlb.40.1.29. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco E. B., Spitzer J. A. Early effects of Escherichia coli endotoxin infusion on vasopressin-stimulated breakdown and metabolism of inositol lipids in rat hepatocytes. Biochem Biophys Res Commun. 1988 Aug 30;155(1):151–159. doi: 10.1016/s0006-291x(88)81062-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco E. B., Spitzer J. A. Eicosanoid production in nonparenchymal liver cells isolated from rats infused with E. coli endotoxin. J Leukoc Biol. 1990 Dec;48(6):488–494. doi: 10.1002/jlb.48.6.488. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco E. B., Spitzer J. A. Impairments in vasopressin-stimulated inositol lipid metabolism in hepatocytes of septic rats. Circ Shock. 1988 Aug;25(4):299–307. [PubMed] [Google Scholar]
- Rodriguez de Turco E. B., Spitzer J. A. Kinetics of diacylglycerol accumulation in response to vasopressin stimulation in hepatocytes of continuously endotoxaemic rats. Biochem J. 1988 Jul 1;253(1):73–79. doi: 10.1042/bj2530073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Turco E., Spitzer J. A. Impairments in hepatocyte phosphoinositide metabolism in endotoxemia. Metabolism. 1987 Aug;36(8):753–760. doi: 10.1016/0026-0495(87)90112-0. [DOI] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Miani N., Rossi F. Ion movement across leukocyte plasma membrane and excitation of their metabolism. Nature. 1975 Feb 13;253(5492):542–544. doi: 10.1038/253542a0. [DOI] [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., van der Meulen J., Brouwer A., Hummel M. J., Mauw B. J., van der Ploeg J. C., Wisse E. Uptake by liver cells of endotoxin following its intravenous injection. Lab Invest. 1981 Jul;45(1):38–45. [PubMed] [Google Scholar]
- Ryan G. B., Majno G. Acute inflammation. A review. Am J Pathol. 1977 Jan;86(1):183–276. [PMC free article] [PubMed] [Google Scholar]
- Schell-Frederick E. Stimulation of the oxidative metabolism of polymorphonuclear leucocytes by the calcium ionophore A23187. FEBS Lett. 1974 Nov 1;48(1):37–40. doi: 10.1016/0014-5793(74)81056-2. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. H., Beger H. G. Sauerstoffradikale im septischen Schock. Chirurg. 1988 Dec;59(12):836–841. [PubMed] [Google Scholar]
- Shiratori Y., Kawase T., Shiina S., Okano K., Sugimoto T., Teraoka H., Matano S., Matsumoto K., Kamii K. Modulation of hepatotoxicity by macrophages in the liver. Hepatology. 1988 Jul-Aug;8(4):815–821. doi: 10.1002/hep.1840080420. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Tanaka M., Hai K., Kawase T., Shirna S., Sugimoto T. Role of endotoxin-responsive macrophages in hepatic injury. Hepatology. 1990 Feb;11(2):183–192. doi: 10.1002/hep.1840110205. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Teraoka H., Matano S., Matsumoto K., Kamii K., Tanaka M. Kupffer cell function in chronic ethanol-fed rats. Liver. 1989 Dec;9(6):351–359. doi: 10.1111/j.1600-0676.1989.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Spitzer J. A., Deaciuc I. V. Effect of endotoxicosis and sepsis on intracellular calcium homeostasis in rat liver. Mitochondrial and microsomal calcium uptake. Circ Shock. 1986;18(2):81–93. [PubMed] [Google Scholar]
- Spitzer J. A., Fish R. E. Lipolytic patterns in isolated adipocytes of continuously endotoxemic rats. Circ Shock. 1986;18(1):21–29. [PubMed] [Google Scholar]
- Spitzer J. A., Friedman H., Newton C., Widen R., Pross S., Klein T. W. Suppressed in vitro blastogenic responsiveness of rat spleen cells after continuous infusion of endotoxin by an implanted osmotic pump. Proc Soc Exp Biol Med. 1987 Oct;186(1):21–26. doi: 10.3181/00379727-186-42578. [DOI] [PubMed] [Google Scholar]
- Spitzer J. A., Nelson K. M., Fish R. E. Time course of changes in gluconeogenesis from various precursors in chronically endotoxemic rats. Metabolism. 1985 Sep;34(9):842–849. doi: 10.1016/0026-0495(85)90109-x. [DOI] [PubMed] [Google Scholar]
- Spitzer J. A., Turco E. R., Deaciuc I. V., Roth B. L. Perturbation of transmembrane signaling mechanisms in acute and chronic endotoxemia. Prog Clin Biol Res. 1987;236A:401–418. [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985 Jan 10;260(1):86–92. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bossuyt H., De Zanger R. B., Wisse E. Cellular and subcellular distribution of injected lipopolysaccharide in rat liver and its inactivation by bile salts. J Hepatol. 1988 Dec;7(3):325–337. doi: 10.1016/s0168-8278(88)80005-9. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Warren J. S., Johnson K. J. Oxygen radicals, inflammation, and tissue injury. Free Radic Biol Med. 1988;5(5-6):403–408. doi: 10.1016/0891-5849(88)90114-1. [DOI] [PubMed] [Google Scholar]


