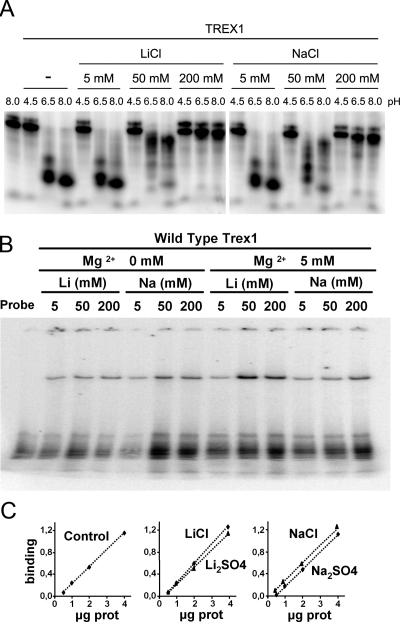
Figure 1.
TREX1 inhibition by lithium and sodium ions. (A) Exonuclease reactions for the in-gel assay were prepared with 100 pg of TREX1 enzyme and 2 nM of a 25-mer oligonucleotide and performed as described in Materials and Methods. The reactions contained increasing concentrations of lithium or sodium chloride and were buffered at a range of pH values, as indicated. (B) TREX1 binds to DNA in the presence of different concentrations of Mg2+, Li, or Na. Recombinant TREX1 was incubated with a radiolabeled probe, and the complexes DNA–protein were separated in an acrylamide gel. (C) Quantitation of TREX binding to DNA in the presence of Li or Na. EMSA assays were performed with increasing amounts of the recombinant protein in the presence of 200 mM of the indicated compounds. The quantification graph indicates the relative binding of the oligonucleotide with respect to the probe alone (arbitrary units).