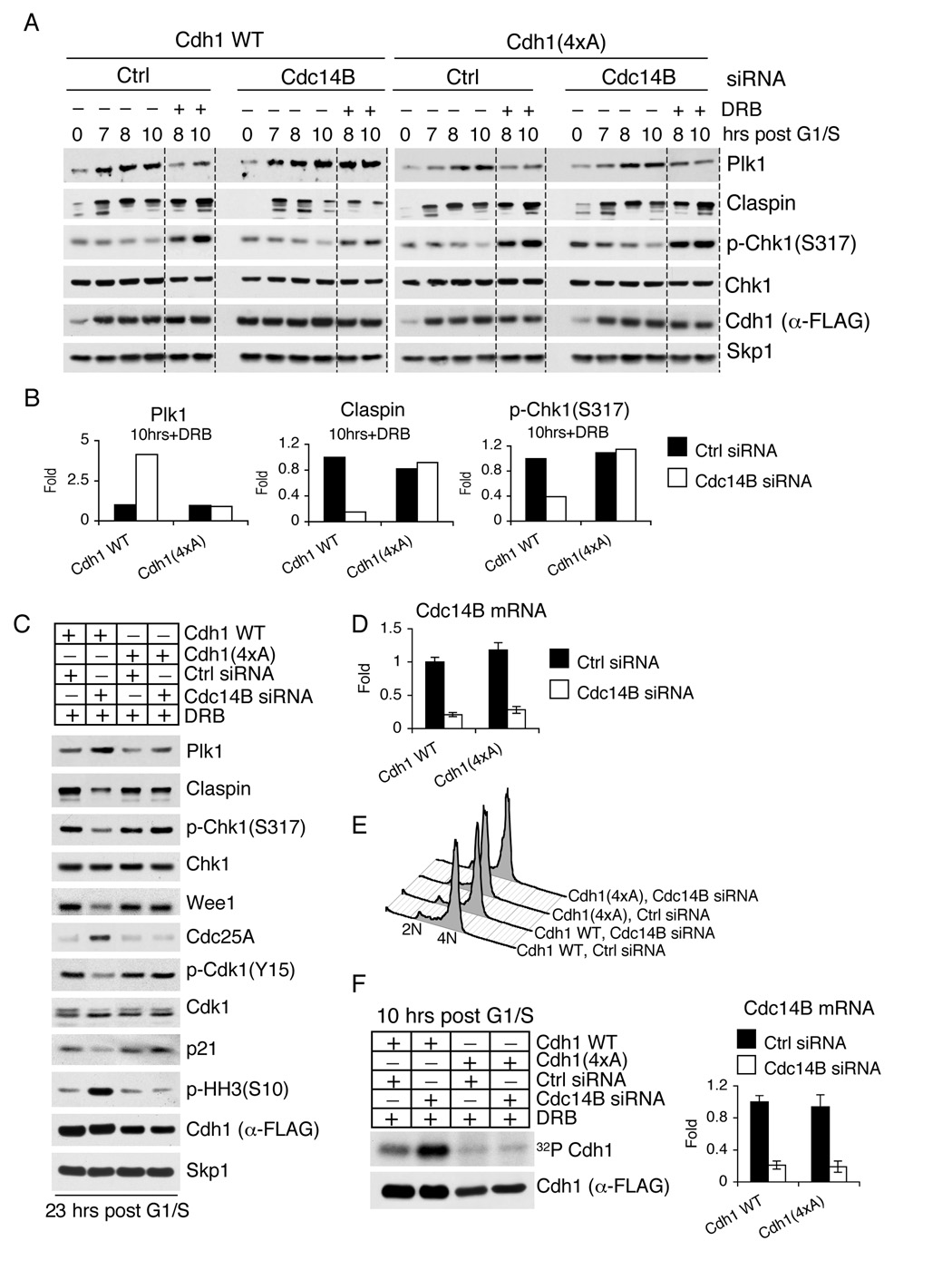
Figure 6. A constitutively active Cdh1 mutant is refractory to the silencing of Cdc14B.
(A) U2OS cells were retrovirally infected with either wild type Cdh1 or Cdh1(4xA) mutant and subsequently transfected with the indicated siRNA oligos. Cells were then synchronized and treated with DRB as described in (2A), collected at the indicated times, and immunoblotted with antibodies to the indicated proteins. To facilitate comparison, a gray line separates samples treated with doxorubicin from untreated samples.
(B) The graphs show the quantification of the levels of Claspin, Plk1, and Chk1 phosphorylated on Ser317 shown in (A) at the indicated timepoint averaged with an additional, independent experiment. The value given for the amount of protein present in the control sample two hours after the end of the doxorubicin pulse was set as 1 (n=2).
(C) U2OS cells expressing wild type Cdh1 or Cdh1(4xA) were transfected with the indicated siRNA oligos and treated as in (A). Twenty-three hours after release from G1/S (16 hours after the doxorubicin pulse), cells were collected, and cell lysates were analyzed by immunoblotting with antibodies to the indicated proteins.
(D)Cdc14B mRNA levels of cells used in (A) and (C) were analyzed eight hours after release from G1/S using real time PCR in triplicate measurements (± SD). The value given for the amount of Cdc14B mRNA present in the sample expressing wild type Cdh1 and treated with control oligos was set as 1.
(E) Synchrony in G2 was ascertained by flow cytometry for cells used in (A) at the time of doxorubicin treatment. Identical synchrony in G2 was obtained for cells used in (C).
(F) The experiment was performed as in (A), except that an in vivo labeling with 32P-orthophosphate was performed during the last three hours before cells were collected. Cdh1 was then immunoprecipitated under denaturing conditions, resolved by SDS-PAGE, and visualized by autoradiography (upper panel) or immunoblotting (bottom panel). Right panel shows Cdc14B mRNA levels analyzed by real time PCR.