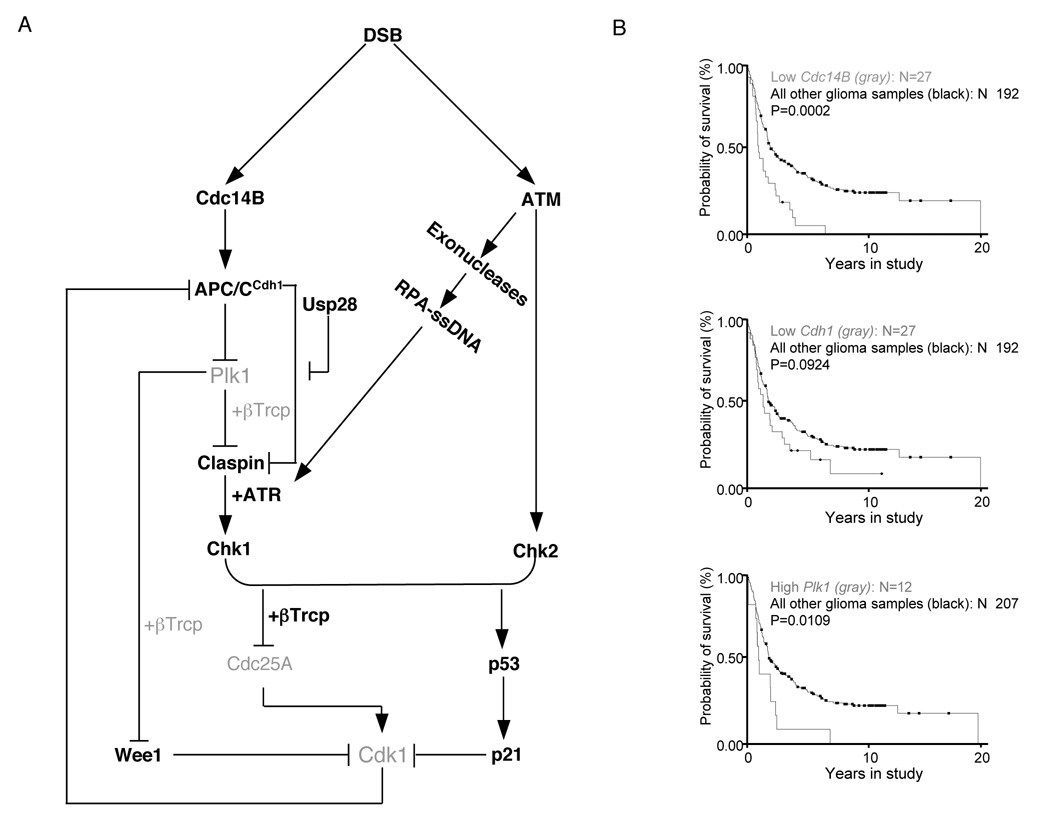
Figure 7. The Cdc14B-Plk1-Cdh1 axis controls the DNA damage response in G2 and is deregulated in human tumors.
(A) A model of the G2 DNA damage response checkpoint. Black signifies activated forms of the respective proteins, and gray indicates inactive forms or degraded proteins. After induction of double strand breaks (DSB) in G2, ATM activates Chk2. In addition, ATM activates certain exonucleases to induce DSB resection, resulting in RPA coated, single-stranded DNA (ssDNA), which contributes to the recruitment of ATR. In parallel, Cdc14B is released from the nucleolus to the nucleoplasm, activating APC/CCdh1, which in turn targets Plk1 for proteasomal degradation. Because of the low levels of Plk1, phosphorylation of Claspin and Wee1 is reduced, preventing βTrcp-mediated degradation. Claspin is protected from APC/CCdh1-mediated degradation by Usp28. Stable Claspin promotes the ATR-mediated activation of Chk1, which, together with Chk2, targets Cdc25A (inducing its degradation) and p53 (promoting its stabilization and consequent induction of p21). As a result, Cdk1 activity is attenuated, and cells arrest in G2. Stable Wee1 contributes to this inhibition by directly phosphorylating Cdk1. The reduction in Cdk1 activity further removes the constraints on APC/CCdh1 activity.
(B) Poor patient survival correlates with low levels of Cdc14B and Cdh1 and high levels of Plk1 (gray).Rembrandt glioma database (http://rembrandt.nci.nih.gov)-generated Kaplan-Meier survival curves of 219 patients with gliomas of all histological grades grouped by gene expression levels of Cdc14B (top plot), Cdh1 (middle plot), and Plk1 (bottom plot). The associated P values are shown for each plot.