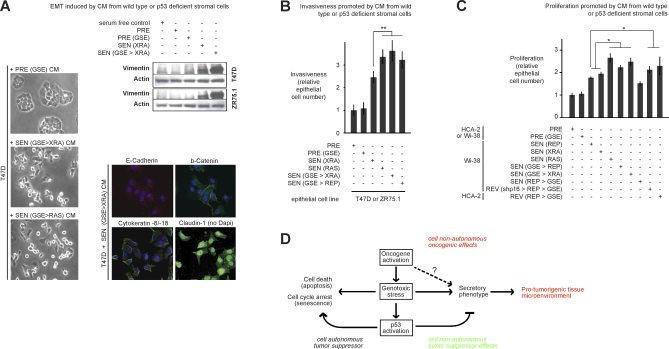
Figure 7. Biological Activities of the Amplified SASP.
(A) T47D and ZR75.1 cells were incubated with the indicated CM for 3 d and then analyzed for cell scattering, immunostained for the indicated proteins, and analyzed for vimentin and actin levels by western blotting. Controls for the immunofluorescence from the same individual experiment are shown in Figure 4B. The senescence inducer is given in parentheses. p53 status was either wild type or deficient (GSE). Manipulations are indicated in sequence, separated by >.
(B) Epithelial cells were incubated with CM from the indicated WI-38 cells and assayed for invasion as described in Materials and Methods and Figure 3C. Double asterisks (**) indicate p < 0.02. Error bars indicate the standard deviation around the mean.
(C) Epithelial cells were incubated with CM from the indicated fibroblasts and cell number was determined by cell counting, total protein, or green fluorescent protein (GFP) fluorescence using epithelial cells expressing GFP, as described in [80]. A single asterisks (*) indicates p < 0.05. Error bars indicate the standard deviation around the mean.
(D) Model for the cell-nonautonomous activities of oncogenic RAS and the p53 tumor suppressor protein. Oncogenic and genotoxic stress of sufficient magnitude to cause senescence induce a SASP, whereby cells secrete inflammatory cytokines, chemokines, and growth factors that can alter the tissue microenvironment and stimulate malignant phenotypes in nearby cells. Thus, in addition to their well-known cell-autonomous effects, oncogenes can promote cancer cell-nonautonomously by inducing a SASP. Oncogenic and genotoxic stress also activate p53, which suppresses cancer by cell-autonomous mechanisms (promoting repair, or inducing apoptosis or senescence). In addition, p53 suppresses cancer by the cell-nonautonomous effects of suppressing the intensity of the SASP and its deleterious effects.