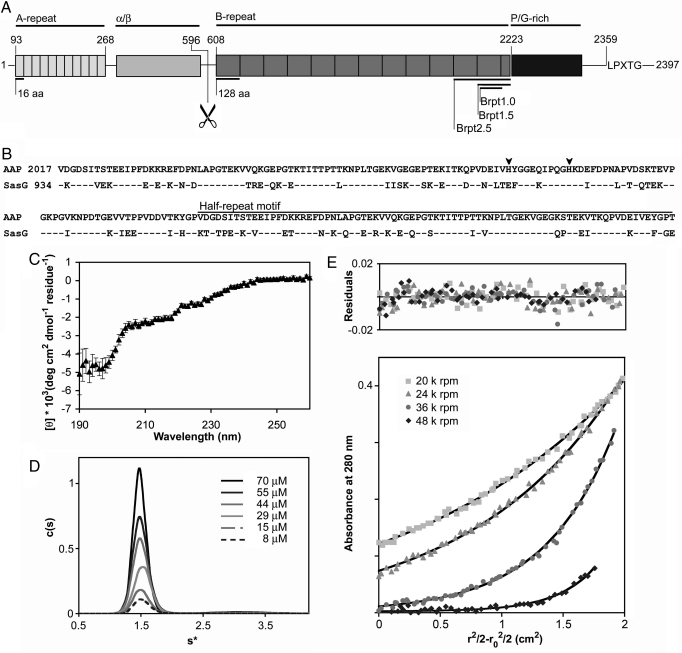
Fig. 1.
Characterization of the G5 domain from Aap. (A) The five regions of Aap are illustrated: the A-repeat region, the putative globular domain (α/β), the B-repeat region containing 5–17 tandem G5 domains, the collagen-like proline/glycine-rich region and a cell wall anchoring motif (LPXTG). The proteolysis site is illustrated with scissors. The domain boundaries of the Brpt1.0, Brpt1.5, and Brpt2.5 constructs are illustrated. (B) Sequence alignment of the terminal G5 domain and C-terminal half-repeat motif from Aap and SasG. Identical amino acids are indicated by dashes (–); histidines are highlighted with arrowheads. Blast alignment (41) shows 80% conservation and 65% identity. (C) Far-UV circular dichroism spectrum of Brpt1.5 in 20 mM Tris pH 7.4, 50 mM NaF. Deconvolution of the data reveals predominantly β-sheet and coil secondary structure elements (Table S1). (D) Sedimentation coefficient distribution plot for Brpt1.5 at varying concentrations. Molecular weight estimation indicates Brpt1.5 is monomeric. (E) Representative sedimentation equilibrium data for Brpt1.5, confirming a monomeric state (Table S3). Black lines show the global fits; residuals are shown above.