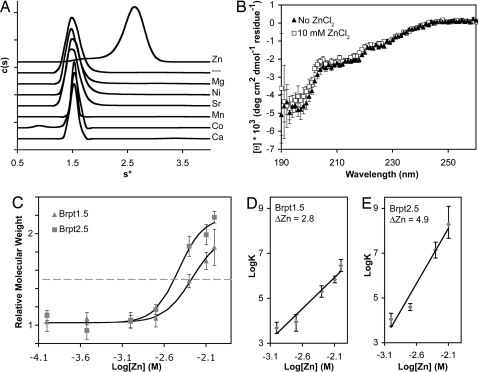
Fig. 2.
Characterization of Zn2+-dependent G5 dimerization. (A) Sedimentation velocity analyses of Brpt1.5 in the presence of divalent cations revealed a Zn2+-specific dimerization event. The dashed line label (––) represents cation-free Brpt1.5. (B) Far-UV CD spectra of Brpt1.5 in the presence (squares) and absence (triangles) of Zn. (C) Sedimentation equilibrium analysis of Zn2+-mediated dimerization of Brpt1.5 (triangles) and Brpt2.5 (squares). Brpt2.5 dimerization requires lower [Zn2+] (EC50 = 3.7 mM) compared to Brpt1.5 (EC50 = 5.4 mM) and shows a steeper slope for the monomer–dimer transition, suggesting enhanced cooperativity of Zn2+ binding with increasing numbers of tandem G5 domains. Error bars show 95% confidence intervals. (D) Linked equilibrium plot of Zn binding by Brpt1.5. Linear regression analysis of the slope indicates that the number of Zn2+ ions taken up upon Brpt1.5 dimerization (i.e., ΔZn2+) is approximately three. (E) Linked equilibrium plot of Zn binding by Brpt2.5. Linear regression analysis of the slope indicates that approximately five Zn2+ ions are bound upon Brpt2.5 dimerization.