Abstract
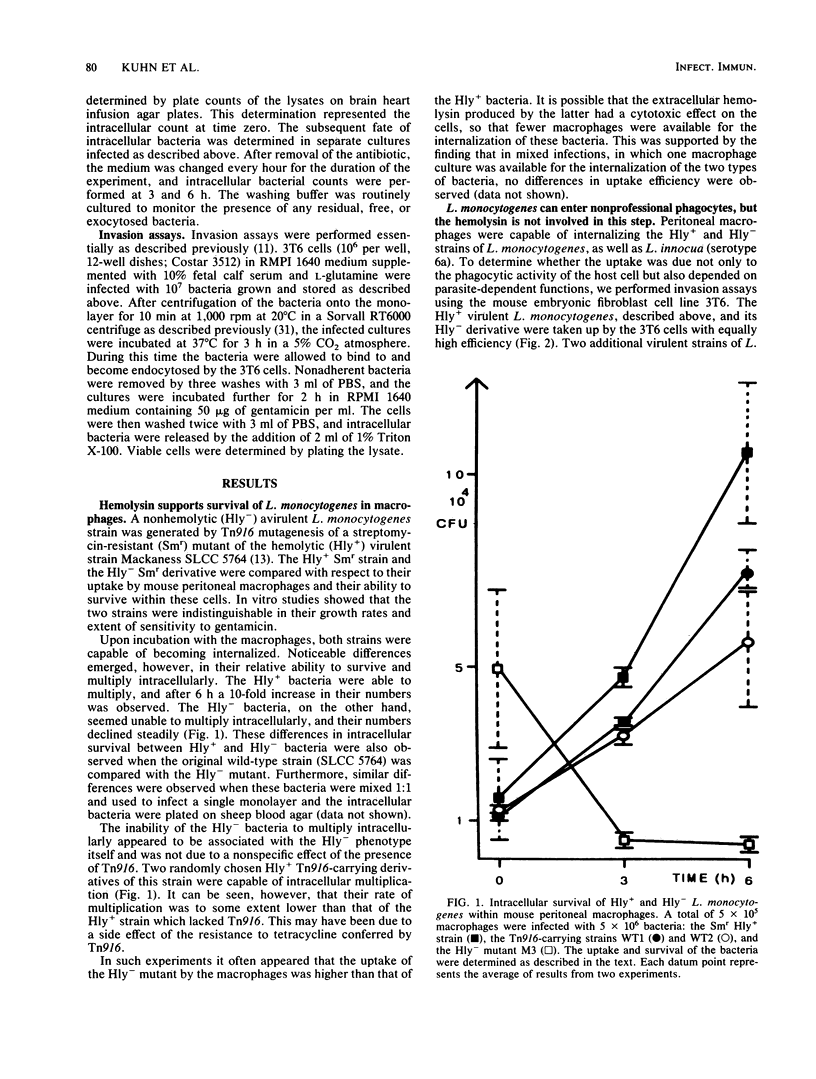
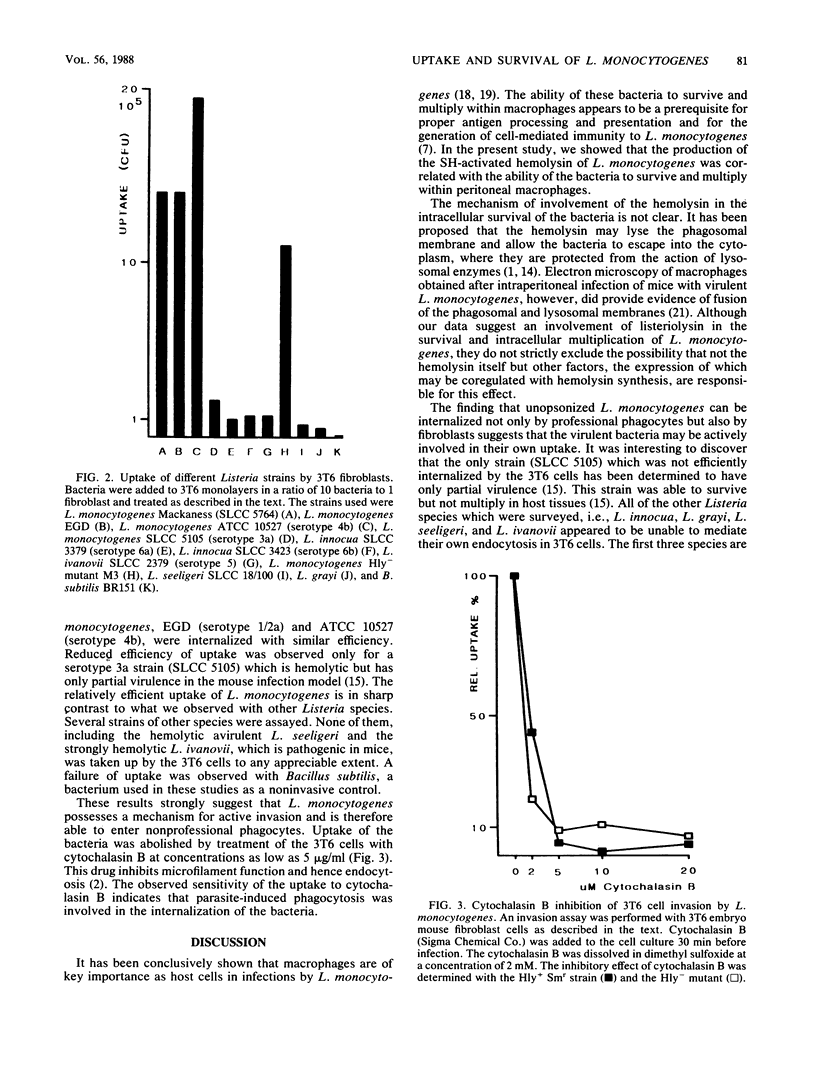
The gram-positive bacterium Listeria monocytogenes is a facultative intracellular pathogen. The only known property of L. monocytogenes which has been shown to be involved in virulence is a hemolysin, listeriolysin (J. L. Gaillard, P. Berche, and P. Sansonetti, Infect. Immun. 52:50-55, 1986; S. Kathariou, P. Metz, H. Hof, and W. Goebel, J. Bacteriol. 169:1291-1297, 1987). Using our previously obtained transposon Tn916-induced hemolysin-negative mutants of L. monocytogenes Sv1/2a (Mackaness strain), we demonstrated that the loss of hemolysin reduced significantly the rate of survival of the bacteria in mouse peritoneal macrophages but did not reduce their uptake. It was further shown that virulent L. monocytogenes strains could invade the mouse embryo fibroblast 3T6 cell line, i.e., mammalian cells which are nonprofessional phagocytes. This uptake was inhibited by cytochalasin B and hence seems to be accomplished by parasite-induced endocytosis. Hemolysin was not essential for this step. Strains of other Listeria species could not efficiently penetrate the 3T6 cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong B. A., Sword C. P. Electron microscopy of Listeria monocytogenes-infected mouse spleen. J Bacteriol. 1966 Mar;91(3):1346–1355. doi: 10.1128/jb.91.3.1346-1355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Allison A. C. Effects of cytochalasin B on endocytosis and exocytosis. Front Biol. 1978;46:143–160. [PubMed] [Google Scholar]
- Devenish J. A., Schiemann D. A. HeLa cell infection by Yersinia enterocolitica: evidence for lack of intracellular multiplication and development of a new procedure for quantitative expression of infectivity. Infect Immun. 1981 Apr;32(1):48–55. doi: 10.1128/iai.32.1.48-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Harrington-Fowler L., Henson P. M., Wilder M. S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981 Jul;33(1):11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R. Observations on Listeria monocytogenes type 5 (Iwanow) isolated in New Zealand. Med Lab Technol. 1973 Jan;30(1):51–56. [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Effects of Listeria monocytogenes Hemolysin on Phagocytic Cells and Lysosomes. Infect Immun. 1970 Apr;1(4):356–362. doi: 10.1128/iai.1.4.356-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorz W., Hof H. Zur Pathogenität von Listerien. Immun Infekt. 1986 Apr;14(2):76–80. [PubMed] [Google Scholar]
- Lepay D. A., Steinman R. M., Nathan C. F., Murray H. W., Cohn Z. A. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985 Jun 1;161(6):1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980 May;28(2):516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod N. S., Watt J. A., Harris J. C. Listeria monocytogenes type 5 as a cause of abortion in sheep. Vet Rec. 1974 Oct 19;95(16):365–367. doi: 10.1136/vr.95.16.365. [DOI] [PubMed] [Google Scholar]
- NORTH R. J., MACKANESS G. B. ELECTRON MICROSCOPICAL OBSERVATIONS ON THE PERITONEAL MACROPHAGES OF NORMAL MICE AND MICE IMMUNISED WITH LISTERIA MONOCYTOGENES. I. STRUCTURE OF NORMAL MACROPHAGES AND THE EARLY CYTOPLASMIC RESPONSE TO THE PRESENCE OF INGESTED BACTERIA. Br J Exp Pathol. 1963 Dec;44:601–607. [PMC free article] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural host. I. Rabbit studies. J Infect Dis. 1954 Jul-Aug;95(1):52–66. doi: 10.1093/infdis/95.1.52. [DOI] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural hosts. II. Sheep studies. J Infect Dis. 1954 Jul-Aug;95(1):67–78. doi: 10.1093/infdis/95.1.67. [DOI] [PubMed] [Google Scholar]
- Rocourt J., Alonso J. M., Seeliger H. P. Virulence comparée des cinq groupes génomiques de Listeria monocytogenes (sensu lato). Ann Microbiol (Paris) 1983 May-Jun;134A(3):359–364. [PubMed] [Google Scholar]
- Rácz P., Tenner K., Mérö E. Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental listeria infection. Lab Invest. 1972 Jun;26(6):694–700. [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Seeliger H. P. Apathogene listerien: L. innocua sp. n. (Seeliger et Schoofs, 1977). Zentralbl Bakteriol Mikrobiol Hyg A. 1981;249(4):487–493. [PubMed] [Google Scholar]
- Seeliger H. P., Schrettenbrunner A., Pongratz G., Hof H. Zur Sonderstellung stark hämolysierender Stämme der Gattung Listeria. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):176–190. [PubMed] [Google Scholar]
- Vesikari T., Bromirska J., Mäki M. Enhancement of invasiveness of Yersinia enterocolitica and Escherichia coli in HEp-2 cells by centrifugation. Infect Immun. 1982 May;36(2):834–836. doi: 10.1128/iai.36.2.834-836.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


