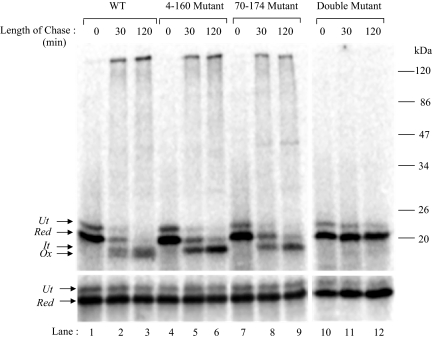
Figure 5.
Folding of RBP-disulfide mutants. WT and the disulfide mutants of RBP, generated as described under Materials and Methods, were translated in a rabbit reticulocyte lysate system supplemented with dog pancreas microsomes under reducing conditions for 60 min at 27°C. Disulfide oxidation was initiated by the addition of 4 mM GSSG, and samples were collected at the indicated chase times. The samples were alkylated with 20 mM NEM and subjected to immunoprecipitation with anti-RBP antibody. The immunoprecipitates were analyzed by 13% nonreducing SDS-PAGE (top) or reducing SDS-PAGE (bottom). WT (lanes 1–3), 4–160 mutant (lanes 4–6), 70–174 mutant (lanes 7–9), and the double mutant (lanes 11–13) are shown. The positions of untranslocated (Ut), reduced (Red), folding intermediate (It), and fully oxidized (Ox) forms are indicated. (Bottom) Analysis of the same samples in reduced form.