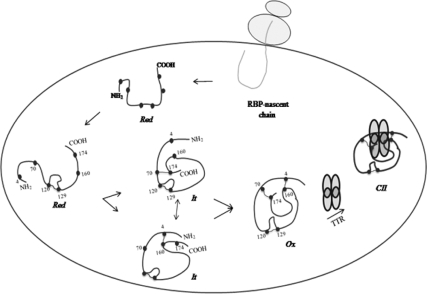
Figure 9.
A model depicting biogenesis of RBP in the ER. Newly synthesized RBP undergoes oxidative folding bound to a pre-existing complex of chaperones. It remains associated with chaperones in the ER until two of its three intramolecular disulfides are formed. On formation of disulfide bonds, RBP dissociates from the chaperones and assembles with a tetramer of TTR in the ER. Cysteines ( ), disulfide bond (
), disulfide bond ( ), and transthyretin monomer (
), and transthyretin monomer ( ).
).