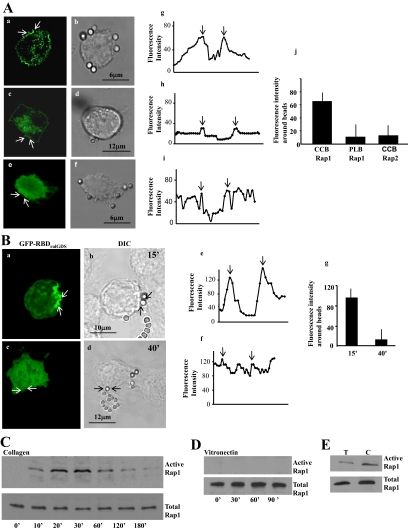
Figure 1.
(A) Localization of GFP-Rap1 in fibroblasts treated with collagen-coated beads (CCB) (a and b) or poly-l-lysine (PLB) beads (c and d), or localization of Rap2 in fibroblasts treated with CCB (e and f). Confocal microscopy line scans of fluorescence intensities across beads showed a >3-fold accumulation of Rap1 around collagen beads compared with poly-l-lysine–coated beads or Rap2 around collagen beads (Figure 1A, g–i). The graphs (g–i) show fluorescence intensity line scans across bead sites marked by the arrows in panels a, c, and e. The arrows in the graphs (g–i) show the point of intersection with beads. Data from 25 separate line scans from three independent experiments is quantified in the histogram (j; mean ± SEM; CCB for Rap1 was larger than PLB or Rap2; p < 0.01). (B) Localization of active Rap1 in fibroblasts treated with collagen-coated beads. GFP-RBD of RalGDS, which binds active Rap1, localized to collagen beads at early times of incubation (a and b), but was diffusely distributed throughout the cell by 40 min after collagen bead incubation (c and d). Fluorescence intensity line scans (e and f) at the bead site marked by arrows in panels a and c. The arrows in graphs e and f show the point of intersection with beads. The histograms g and j show the fluorescence intensity (mean ± SEM) from three independent experiments, 25 cells per experiment. (C and D) A pulldown assay with GST-RalGDS to determine levels of GTP-bound Rap1 associated with collagen or vitronectin beads. Data shown are representative of three independent experiments. (E) Cells incubated with α2β1-blocking antibody (T) or with control antibody (C) were stimulated with collagen beads, and active Rap1 was determined by immunoblotting of RalGDS-binding proteins. This experiment was repeated three times with similar results.