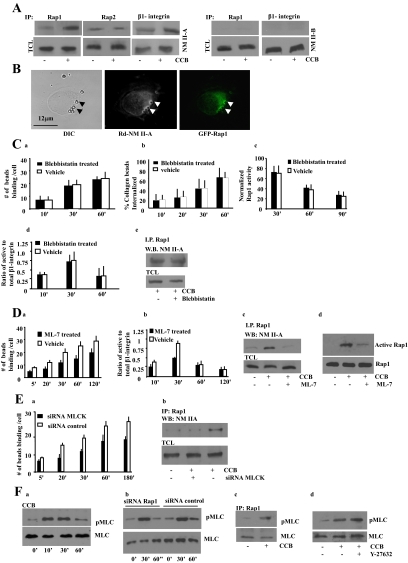
Figure 3.
(A) Cells were incubated with collagen coated beads (CCB; +) or not (−). Rap1, Rap2, or β1-integrin immunoprecipitates of bead-associated proteins were immunoblotted for NM II-A or NM II-B. TCL, total cell lysates. (B) Colocalization of NM II-A and GFP-Rap1 at bead sites. The overlay shows close spatial association of Rap1 and NM II-A at bead sites. (C, a and b) Fibroblasts treated with vehicle or 25 μM blebbistatin exhibit similar collagen-bead binding and collagen-bead internalization. Similar results were obtained with 50 or 100 μM blebbistatin. (c–e) Cells incubated with 25 μM blebbistatin did not effect collagen-induced Rap1 activity, β1-integrin activation, or Rap1 interaction with NM II-A. (D) (a) ML-7– treated (25 μM) fibroblasts show reduced collagen-bead binding (p < 0.01 after 5 min). (b) Ratio of active to total β1-integrin around collagen beads in ML-7 and vehicle-treated cells. ML-7 disrupts the interaction of NM II-A with Rap1. (c) Rap1 immunoprecipitates in presence or absence of ML-7 treatment, and collagen induced samples were probed with NM II-A antibody. CCB, collagen-coated beads; TCL, total cell lysate. (d) Pulldown assays using GST-RalGDS to evaluate the effect of ML-7 treatment on Rap1 activity in cells stimulated with collagen. (E) (a) MLCK knockdown cells exhibit reduced collagen-bead binding (p < 0.01 after 5 min). (b) Rap1 immunoprecipitates were isolated from cells transfected with siRNA to MLCK after previous incubation with collagen beads (30 min). Blots were probed for NM II-A. For C, a and b; D, a and b; and E, panel a, data represent the mean ± SEM for three independent experiments, 25 cells per experiment. (F) (a) Bead-associated proteins collected from cells induced with collagen-coated beads show maximal phosphorylation of MLC at 30 min. (b) Cells transfected with Rap1 siRNA show similar MLC phosphorylation during incubation with collagen-coated beads as siRNA controls. (c) Rap1 immunoprecipitates interacted with phospho-MLC in response to collagen-bead binding. (d) Treatment with Rho kinase inhibitor (Y-27632; 10 μM) does not effect phosphorylation of MLC in response to collagen binding.