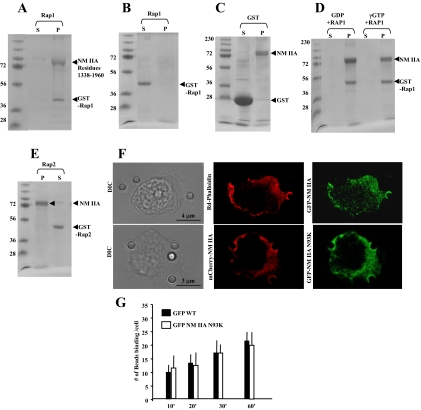
Figure 4.
(A) GST-Rap1 (8 μM) binding to assembled NM II-A rods (10 μM) in a standard pelleting assay. GST-Rap1 is in the pellet with the assembled NM II-A rods. (B) GST-Rap1 alone in pellet (P) and supernatant (S). (C) GST alone does not bind to NM II-A rods (12 μM GST and 8 μM NM II-A rods). (D) GST-Rap1 (8 μM) was incubated with 140 μM GTPγS or GDP in buffer containing 50 mM Tris, pH 7.4, 1 mM EDTA, 20 mM NaCl, and 1% Triton X-100 for 10 min at room temperature followed by GTPγS or GDP. After 30 min, samples were precleared with 50 μl glutathione-Sepharose before incubation with assembled NM II-A rods. (E) GST-Rap2 (8 μM) shows no interaction with filamentous NM II-A rods. (A–D) S, supernatant; P, pellet. (F) Top panel, representative images showing distribution of GFP-NM II-A filaments and F-actin after Triton permeabilization. A total of 20 cells were examined. Bottom panel: Images show colocalization of WT mCherry-NM II-A with N93KGFP-NM II-A mutant lacking motor activity in Triton-permeabilized cells. (G) There was no difference in collagen-bead binding in cells transfected with WT GFP -NM II-A or GFP NM II-A N93K.