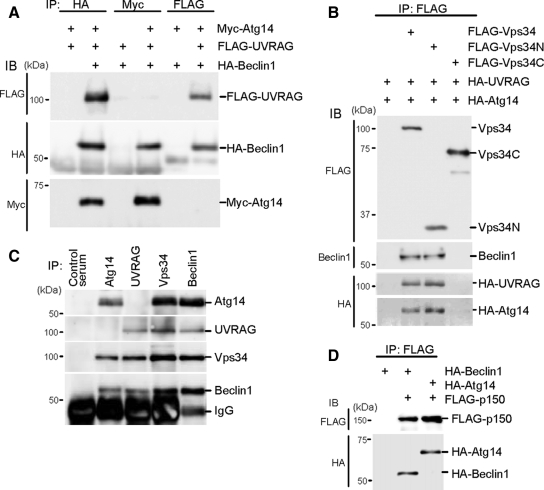
Figure 2.
Atg14 and UVRAG form two distinct complexes with Beclin 1-Vps34. (A) Human Atg14 and UVRAG are included in different Beclin 1 complexes. HEK293T cells were cotransfected with FLAG-UVRAG, HA-Beclin 1, and Myc-Atg14, and cell lysates were subjected to immunoprecipitation using antibodies against HA, Myc, and FLAG. The resulting precipitates were examined by immunoblot analysis with the indicated antibodies. (B) Atg14 and UVRAG interacts with the N-terminal C2 domain of Vps34. HEK293T cells were cotransfected with HA-UVRAG, HA-Atg14, and either FLAG-Vps34 (full-length), FLAG-Vps34N (containing C2 domain, 1-282 amino acid residues), or FLAG-Vps34C (283-887 amino acid residues) and analyzed as described in A. (C) Endogenous Atg14 and UVRAG form protein complexes with Vps34 and Beclin 1 but not with each other. Lysates of HEK293T cells were used for immunoprecipitation with indicated antiserum. (D) Atg14 forms a protein complex with p150. Lysates of HEK293T cells transiently expressing FLAG-p150 and either HA-Beclin 1 or HA-Atg14 were analyzed by immunoprecipitation and immunoblotting.