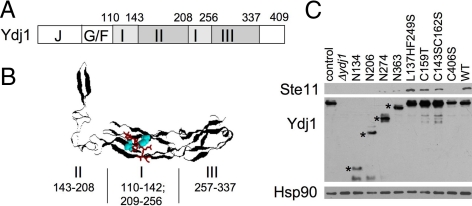
Figure 1.
Effect of Ydj1 alteration on Ste11 accumulation. (A) Schematic of the domain structure of Ydj1. The amino acid sequence encoding domain I is split by the sequences encoding domain II (the zinc-binding domain). (B) A ribbon diagram of the x-ray crystal structure of the SBD of Ydj1 (amino acids 110-337) cocrystallized with the GWLYEIS peptide (Li et al., 2003). The residues comprising each domain are shown below the structure. The GWLYEIS peptide is depicted in red stick mode and the L137 and F249 residues are aqua in space filling mode. The figure was generated with RasMol (PDB file 1NLT). (C) Full-length WT Ste11 was constitutively expressed in a ydj1::HIS3 strain expressing the indicated ydj1 mutation. His-Ste11 was isolated by incubation of cell lysate with nickel resin. Control sample expressed WT YDJ1 but lacked plasmid expressing His-Ste11. Top, His-Ste11was eluted from nickel resin in SDS-sample buffer, separated by SDS-PAGE and immunoblotted with anti-Xpress (His-Ste11). Middle and bottom, whole cell lysate was immunoblotted with antibodies specific for Ydj1 or Hsp90. The truncated forms of Ydj1 are indicated with an asterisk (*).