Abstract
Galectins, a family of β-galactoside binding lectins, have recently emerged as novel regulators of tissue homeostasis. Galectin-7 is predominantly expressed in stratified epithelia, especially in epidermis. We report here the generation of galectin-7–deficient mice that are viable and do not display phenotypical abnormalities in skin structure or expression of epidermal markers. However, these mice show unique defects in the maintenance of epidermal homeostasis in response to environmental challenges. First, after UVB irradiation in vivo, the apoptotic response is prematurely triggered and lasts longer in the mutant epidermis. This result contrasts with the proapoptotic role that had been proposed for galectin-7. Second, wound-healing experiments in vivo revealed that galectin-7–deficient mice displayed a reduced reepithelialization potential compared with wild-type littermates. This effect could be attributed to a defect in cell migration. Because galectin-7 is located in the podosomes of keratinocytes migrating out of skin explants in culture, we propose that this glycan-binding protein may directly influence cell/extracellular matrix interactions. Finally, we also detected an unexpected intense hyperproliferative reaction consecutive to both types of stress in galectin-7–deficient mice. Together, these studies provide the first genetic evidence showing that galectin-7 can modulate keratinocyte apoptosis, proliferation, and migration during skin repair.
INTRODUCTION
Galectins are a family of soluble lectins sharing a unique carbohydrate recognition domain (CRD) that confers specificity for β-galactoside derivatives (Barondes et al., 1994a,b). The 15 mammalian members known to date can be classified into three groups on the basis of their structure, namely, “proto-type” galectins containing one CRD domain (14 kDa); galectin-3 (30 kDa), which is a unique chimeric molecule containing a single CRD motif combined with a proline-rich N-terminal domain; and tandem-repeat galectins that are composed of two CRDs separated by a short linker sequence (30 kDa) (Hirabayashi and Kasai, 1993). Despite their lack of signal peptide, galectins can be secreted by an endoplasmic reticulum (ER)-Golgi-independent pathway (Lindstedt et al., 1993; Sato et al., 1993). They can be found both intracellularly (cytoplasm and/or nucleus) and extracellularly depending on the cell type, cell cycle stage, and differentiation state. Galectins can be associated with the plasma membrane, but they do not contain transmembrane domain. Consistent with this variable subcellular location, galectins have been implicated in a wide range of cellular processes, including cell–cell interactions, extracellular matrix remodelling, apoptosis, the cell cycle, intracellular trafficking, and splicing (Hughes, 2001; Liu et al., 2002; Hsu and Liu, 2004; Delacour et al., 2006; Elola et al., 2007). Their roles in cancer biology (Lahm et al., 2004; Takenaka et al., 2004; Liu and Rabinovich, 2005; Thijssen et al., 2007), immune response (Sato and Nieminen, 2004; Rabinovich et al., 2007), and morphogenesis (Poirier, 2002) are well documented for the most studied members of the family. In addition, recent evidence indicates that galectin expression and function are highly sensitive to environmental stress resulting from neuroendocrine and immunological stimuli (Blois et al., 2007; Plachta et al., 2007; Toscano et al., 2007), suggesting a critical role of these glycan-binding proteins in maintaining and restoring tissue homeostasis after different insults.
Galectin-7 is a prototype galectin that forms homodimers (Leonidas et al., 1998). Thus, like other prototype members, galectin-7 can act as a bridging molecule between glycoproteins or glycolipids containing similar glycan structures. Galectin-7 was first isolated in two independent differential screens, one screen searching for human epidermal genes responsive to retinoic acid (Magnaldo et al., 1995), and the other screen for genes down-regulated in actively dividing human keratinocytes transformed in vitro (Madsen et al., 1995). Galectin-7 is preferentially expressed in stratified epithelia, including epidermis, cornea, oral cavity, esophagus and anorectal epithelium (Magnaldo et al., 1998) and major changes in its level of expression have been observed in various types of cancer, most notably in skin tumors (Saussez and Kiss, 2006).
Several lines of evidence indirectly suggested that galectin-7 might play a role in epithelial tissue response to environmental stimuli. First, galectin-7 could intervene in the process of wound-healing because it is up-regulated in wounded cornea (Cao et al., 2003) and addition of the recombinant protein accelerates the speed of healing in culture (Cao et al., 2002). Second, galectin-7 has been described as a proapoptotic factor involved in the epidermal response after UVB injury. Hence, following an early report in which galectin-7 was identified as one of the first genes responding to overexpression of the p53 tumor suppressor gene in DLD-1 human colon cancer cells (Polyak et al., 1997), Bernerd et al. (1999) reported 1) an increased galectin-7 expression in the apoptotic sunburn keratinocytes of human skin explants exposed to UVB and 2) the appearance of apoptotic cells in cultures of transfected human keratinocytes overexpressing galectin-7. Finally, ectopic expression of galectin-7 also renders HeLa cells or DLD-1 cells more sensitive to apoptosis induced by a variety of stimuli in addition to UVB (Kuwabara et al., 2002).
To elucidate the role(s) of galectin-7 in vivo, we have generated galectin-7 null mutant mice and focused our analysis on the consequences of this mutation in adult epidermis. The epidermis is a self-renewing stratified epithelium, which provides a protective barrier that serves multiple key functions, including protection from dehydration, infections, and various environmental insults. We present here the first evidence, using null mutant mice, showing that galectin-7 is involved in maintaining epidermal homeostasis in response to major challenges, i.e., UVB irradiation and wounding.
MATERIALS AND METHODS
Galectin-7 Null Mice
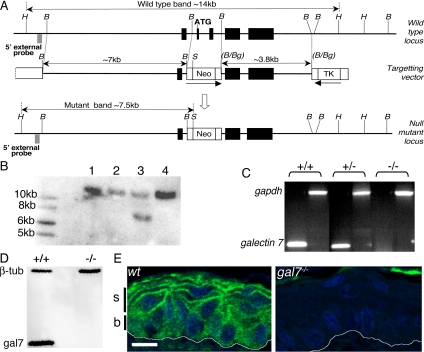
A bacterial artificial chromosome clone containing the mouse galectin-7 gene was obtained from Research Genetics (Huntsville, AL) (clone 254C6, catalog no. 96022). The 3.8-kb BamHI–BamHI fragment containing exons 4 and 5 was subcloned into the BglII site of pKO Scrambler NKTV-1906 (Stratagene, La Jolla, CA). The 7-kb BamHI–BamHI fragment containing exon 1 was then inserted into the BamHI site, making the final targeting vector. Vector DNA was linearized with Not1 and electroporated into WW6 embryonic stem (ES) cells (Ioffe et al., 1995). G418 and gancyclovir doubly resistant clones were screened by Southern blotting. Genomic DNA was digested with HindIII/SpeI and hybridized first with neo probe and then with a specific 5′ external probe (Figure 1, A and B). Chimeric males were obtained by injection of a positive clone into C57Bl/6 blastocysts. Heterozygous individuals were intercrossed, and the mutation was maintained on mixed genetic background (50% 129/Sv; 50% C57Bl/6). We also introduced the galectin-7−/− mutation into the C57Bl/6 background by a series of eight backcrosses. Mice were kept in a specific pathogen-free animal house facility and handled respecting the French regulations for animal care.
Figure 1.
Establishment of galectin-7 null mutant mice. (A) Targeting strategy. Mouse galectin-7 gene comprises five exons (black boxes). The ATG initiation codon is located in exon 2. The 7-kb fragment of 5′ homology contains exon 1, and the 3.8-kb fragment of 3′ homology contains exons 4 and 5 (see Materials and Methods). H, HindIII; B, BamHI; S, SpeI; Bg, BglII; TK, thymidine kinase gene; and Neo, neomycin resistance gene. (B) Southern blot. G418/Gancyclovir double resistant ES cell clones were picked. Genomic DNA was digested with HindIII/SpeI. The wt (14-kb) and targeted (7.5-kb) fragments were detected with a specific 5′ external probe (A). Clone 3 was used in all subsequent experiments. (C) RT-PCR analysis. Skin RNA was prepared from 2-mo-old littermates obtained by crossing heterozygous animals. Galectin-7 mRNA was amplified using the gal7L/gal7R primers (300-bp product). Control amplification using gadph mRNA was done using gapdh5′/gapdh3′ primers, which gave the expected 1193-bp product (see Materials and Methods). (D) Western blot. Fifteen micrograms of protein extracts prepared from foot pads were loaded on 12% acrylamide gel. After blotting, galectin 7 (14-kDa) and β-tubulin (55-kDa) proteins were detected. (E) Galectin-7 distribution in adult epidermis. Paraffin sections from back skin samples of wt and galectin-7−/− mice were immunostained with anti-galectin-7 Ab. White lines indicate the dermoepidermal junction. B, basal layer; s, suprabasal layers. We noted autofluorescence in cornified layer. Bar, 10 μm.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA from adult skin was prepared using RNAPlus2 kit (Qbiogene, Irvine, CA) and cDNA was synthesized with random primers (kit K1403-1; Clontech, Mountain View, CA). Galectin-7 transcripts were detected with 5′-CCATGTCTGCTACCCAGCAC-3′ (gal7L) and 5′-CCTTGAAGCCTTCCTCTGTG-3′(gal7R) primers, respectively, overlapping exon 2–3 and exon 4–5 junctions. As a control, glyceraldehyde-3-phosphate dehydrogenase cDNA was amplified with 5′-GTCCCGTAGACAAAATGGTGAAGG-3′ (gadph5′) and 5′-GGTGCAGCGAACTTTATTGATGG-3′ (gadph3′) primers.
Western Blot Analysis
Protein extracts from two anterior foot pads of adult mice were prepared in 400 μl of lysis buffer (Bernot et al., 2004). Fifteen microliters of each preparation was loaded on to a 12% acrylamide gel. Mouse monoclonal anti-β-tubulin antibody (Ab) was first used (1:8000, Amersham N357; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) and detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse Ab (1:15,000, A9044, Sigma-Aldrich, St. Louis, MO), then rabbit anti-galectin-7 Ab (Magnaldo et al., 1995) was added, followed by HRP-conjugated goat anti-rabbit (1:10,000; Amersham NA934). Signal was revealed using ECL Plus detection system (GE Healthcare RPN2132).
Electron Microscopy
Median back skin samples of 2-mo-old wild-type (wt) and galectin-7−/− mice were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 1 h at room temperature followed by 1% osmium for 1 h at 4°C and contrasted with uranyl acetate before embedding in Epon.
Immunostaining of Skin Sections
Median back skin samples from shaved or depilated mice (UVB experiment) were fixed overnight at 4°C with 4% formaldehyde in phosphate-buffered saline (PBS) before embedding in paraffin (Paraplast). Anterior adult foot pads were embedded in Tissue-Tek OCT (Sakura Finetek, The Netherlands) and immediately snap frozen in liquid N2 before storing at −80°C. All stainings were performed using standard protocols, except for Ki67, which required a preliminary unmasking step at 98°C for 30 min in pH 6.0 solution (S1700; Dako, Trappes, France).
Primary Abs.
Primary antibodies were as follows: anti-galectin-7 rabbit Ab (1:3000; Magnaldo et al., 1995), anti-Ki67 rabbit Ab (1:250, NCL-Ki67p; Novocastra, New Castle, United Kingdom), anti-keratin5 rabbit Ab (1:2500, PR-B160-P; Covance Research Products, Princeton, NJ), anti-keratin10 mouse monoclonal antibody (mAb) (1:2000, C-7284; Sigma-Aldrich), anti-loricrin rabbit Ab (1:200, PRB-145P; BAbCO, Richmond, CA), anti-E-cadherin mouse mAb (1:500, C20820; BD Biosciences, San Jose, CA), anti-β-catenin mouse mAb (1:500, C19220; BD Biosciences), anti-α6-integrin rat mAb (1:50, MAB1378; Millipore, St-Quentin-en-Yvelines, France), anti-desmoglein mouse mAb (1:10, 03-61002; American Research Products, Belmont, MA), anti-desmocollin1 rabbit Ab (1:2000, NB600-666; Novus Biologicals, Littleton, CO), anti-plakoglobin rabbit Ab (1:25, C2069-60A; U.S. Biological, Swampscott, MA), and anti-cortactin monoclonal Ab (1:200, 05-180; Millipore).
Secondary Abs.
Secondary Abs were as follows: Alexa488-conjugated goat anti-rabbit Ab (1:500, A11008; Invitrogen, Cergy Pontoise, France), Alexa568-conjugated goat anti-mouse Ab (1:500, A11004; Invitrogen); and biotinylated goat anti-rat Ab (1:500, 112-065-003; Jackson ImmunoResearch Laboratories, West Grove, PA) followed by Alexa568-conjugated streptavidin (1:500, S11226; Invitrogen).
In Vivo UVB Irradiation
UVB irradiation was done using Philips TL20W/12 fluorescent tubes (LumièreService, Paris, France), with an emission peak at 312 nm (Bernerd et al., 1999). A Kodacel filter (Eastman Kodak, Rochester, NY) blocking wavelengths below 305 nm was added and the irradiance was monitored with a Centra dosimeter (Osram, Berlin, Germany) placed 20 cm away from the source. Back hair of 2-mo-old females (129/Sv; C57Bl/6 background) was removed with depilatory cream under general anesthesia. Five days later, the posterior region of the back was exposed to a single dose of 2000 J/m2 of UVB under general anesthesia. The depilated unirradiated anterior region was used as a control. Mice were killed at different times after UVB irradiation and skin samples (1 cm × 0.5 cm) were taken from the median part of posterior and anterior regions.
Detection of Apoptotic Cells
Apoptotic sunburn cells were identified on paraffin sections stained with hematoxylin and eosin (Kerr et al., 1972). Alternatively, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was carried out using the In Situ Cell Death Detection kit (Roche Diagnostics, Base, Switzerland).
For each animal, the analysis used the entire 1-cm section from two separate slides, spaced 40 sections apart. Results were expressed as the number of apoptotic cells per centimeter of epidermis. This analysis was restricted to the interfollicular regions.
In Vivo Wounding Experiment
Adult mice were anesthetized before a superficial scratch was made along the sagittal axis of tail by using a sterile blood lancet (Assistant, Sondheim, Germany). The animals were killed at 24 or 48 h after injury. Three segments (0.5 cm) of each injured tail were snap-frozen in Tissue-Tek OCT and sectioned (10 μm) for histological analysis and immunostaining. The distance between wound margins was measured after staining with anti-keratin17 rabbit Ab (1:5000; gift from P. Coulombe, Johns Hopkins University, Baltimore, MD). All results are expressed as a mean of three values per individual.
Ex Vivo Wound-healing Assay
Skin explant cultures of 1.5-d-old C57Bl/6 wild-type and mutant mice were performed as described previously (Mazzalupo et al., 2002), except that the biopsies were obtained from ventral skin instead of dorsal skin. Cultures were maintained in medium as described previously (Rheinwald and Green, 1975), fixed on day 7, and stained with anti-keratin17 rabbit Ab (1:5000; gift from P. Coulombe) (Mazzalupo et al., 2002). The surface area of the keratin17-positive outgrowth was quantified. Some explants were treated for 2 h with mitomycin C (10 μg/ml, M0503; Simnga-Aldrich) on day 2, and then they were placed back in normal medium until day 7. For immunofluorescence staining, explants were cultured on glass coverslips for 7 d, fixed in 4% paraformaldehyde for 15 min and then permeabilized with 0.025% saponin in PBS for 20 min. Primary Abs included the following: anti-galectin-7 rabbit Ab (1/3000; Magnaldo et al., 1995), anti-β-tubulin mouse mAb (1:1000), anti-cortactin mouse mAb (1:200, 05-180; Millipore), and anti-lysosome–associated membrane protein (Lamp)-1 mouse mAb (1:50; a gift from H.-P. Hauri, University of Basel, Basel, Switzerland) overnight at 4°C. For β1-integrin staining, the primary goat anti-β1-integrin Ab (1:50, AF2405; R&D Systems Europe, Abingdon, Oxfordshire, United Kingdom) was added in culture medium for 1 h 30 min before fixation. Secondary Abs were as follows: Alexa488-conjugated goat anti-rabbit Ab (1:500, A11008: Invitrogen), Alexa568-conjugated goat anti-mouse Ab (1:500, A11004; Invitrogen), and Alexa568-conjugated donkey anti-goat Ab (1:500, A11057; Invitrogen).
Quantification of Cell Proliferation and Epidermal Thickness
After UVB irradiation, Ki67-positive cells were counted along the entire 1-cm section of epidermis from two separate slides, spaced 40 sections apart. Data are expressed as a number of Ki67-positive cells per centimeter of epidermis. After wounding, Ki67-positive cells were counted over a distance corresponding to one 20× field on either side of the wound.
Epidermal thickness was measured between the basal membrane and the surface of granular cells. Thirty-three measurements were done along the 1-cm section of epidermis. These analyses were restricted to interfollicular regions.
Statistics
The data are expressed as the mean ± SD. The nonparametric Mann–Whitney U-test was used for comparisons (Software StatEL, ad Science, Paris, France).
RESULTS
Galectin-7 Null Mutant Mice Are Viable and Do Not Display Obvious Skin Defects
To inactivate the galectin-7 gene in mice, we designed a targeting vector to delete exons 2 and 3 of the endogenous locus (Figure 1A). ES clones containing the disrupted galectin-7 allele were identified by Southern blot analysis of genomic DNA (Figure 1B). Heterozygous mice obtained after blastocyst injection of one of these clones were intercrossed, and we confirmed that galectin-7 transcripts and protein were absent in homozygous animals (Figure 1, C–E). The null mutant (galectin-7−/− )individuals are found at mendelian ratios (25% null, 21% wild-type, 54% heterozygous; N = 209) showing that the mutation is viable. The mice live and reproduce normally in animal house conditions, and observations over 18 mo revealed no obvious abnormalities in their aspect or general behavior.
In wt adult epidermis, galectin-7 is present in both basal and suprabasal (spinous and granular) cells, but its subcellular distribution differs between the two compartments (Figure 1E). In basal cells, staining is uniformly cytoplasmic, whereas in suprabasal cells, there is a strong, irregular, punctiform signal associated with the plasma membranes. No galectin-7 immunoreactivity is detected in null mutant epidermis (Figure 1E).
We looked more carefully at proliferation and differentiation in the galectin-7 null mutants. Using Ki67 as a marker of dividing cells, we found that the mitotic index was similar between wt (115 ± 14 cells/cm) and galectin-7−/− (116 ± 23 cells/cm) epidermis. Consistent with this, the epidermal thickness is comparable between wt tissue (13.9 ± 1.1 μm; N = 3) and galectin-7−/− tissue (13.1 ± 1.3 μm; N = 3).
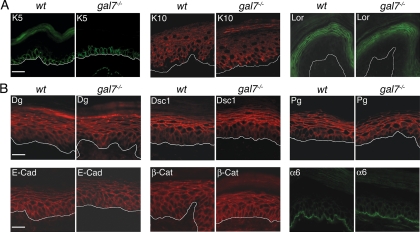
In addition, the differentiation program does not seem to be affected either in the null mutant epidermis, because the markers for the basal (keratin5), suprabasal (keratin10), and granular (loricrin) layers are all normally expressed (Figure 2A). The distribution of the junction molecules, E-cadherin, β-catenin, α6-integrin, and of the desmosomal components desmoglein, desmocollin-1 and plakoglobin, is also unaffected by the lack of galectin-7 and the intensity of the signals is comparable between wt and galectin-7−/− samples (Figure 2B).
Figure 2.
Expression of differentiation and junctional markers in wt and null mutant adult epidermis. Cryosections (5 μm) of anterior foot pads from adult wt and galectin-7−/− mice were stained. (A) Differentiation markers: keratin5 (K5) specific for basal cells, keratin10 (K10) specific for suprabasal cells and loricrin (Lor) specific for superficial granular layers. (B) Junctional markers E-cadherin (E-Cad), β-catenin (β-Cat), α6-integrin (α6), desmoglein (Dg), desmocollin1 (Dsc1), and plakoglobin (Pg). White lines indicate the dermoepidermal junction. Bar, 20 μm.
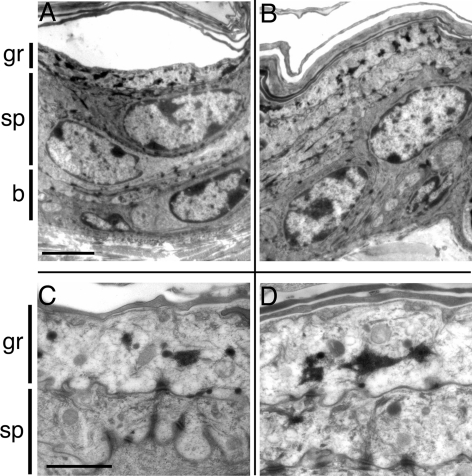
By electron microscopy, we found that the overall organization of the tissue, the intensity of cytoplasmic staining, the shape of nuclei and the amount of heterochromatin, as well as the type and distribution of keratohyalin grains are all similar between wt and galectin-7−/− skin. There is no alteration in desmosome size (0.22 ± 0.07 μm in wt [N = 130] vs. 0.20 ± 0.07 μm in galectin-7−/− [N = 149]) or in cellular interactions (Figure 3).
Figure 3.
Ultrastructure of wt and null mutant adult epidermis. The wt (A and C) and galectin-7−/− (B and D) epidermises were examined by electron microscopy. (A and B) Comparison of the overall organization of the tissue. There is no detectable difference between wt and mutant tissue. Cellular interactions seem normal. Bar, 3 μm. (C and D) High magnification of the superficial layers. The size of desmosomes does not seem affected in the mutant. The distribution and shape of keratohyalin grains are similar between wt and mutant epidermis. Bar, 1 μm. b, basal layer; sp, spinous layer; and gr, granular layer.
Post-UVB Epidermal Response Is Aberrant in Galectin-7–deficient Mice
Depilated back skin of wt and galectin-7−/− adult mice was exposed to a single dose of UVB (2000 J/m2) to examine the apoptotic and regenerative phases triggered after injury.
Apoptotic Response.
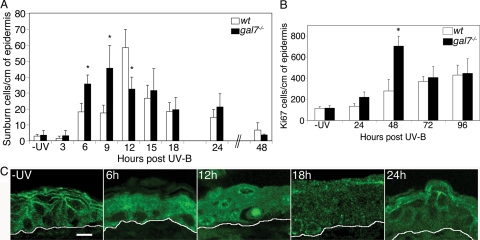
In wt animals, the number of apoptotic cells peaked at 12 h post-UVB, with ∼60 sunburn cells per centimeter of epidermis. This elevated level of apoptosis was limited to the narrow time frame between 9 and 15 h post-UVB, and it returned to basal levels before 48 h. In galectin-7−/− epidermis, we found twice as many sunburn cells as in wt epidermis at the early time points, i.e., 6 and 9 h post-UVB (p < 0.01) (Figure 4A). This was confirmed by TUNEL assay, which also detected a twofold increase in apoptotic cells in galectin-7−/− (58.9 ± 23 cells/cm) compared with wt (26.6 ± 4.7 cells/cm) epidermis at 6 h. After the 6- to 9-h initial phase, the level stabilized until 15 h post-UVB exposure of mutant mice. Thus, unlike wt epidermis, galectin-7−/− epidermis displayed no distinct narrow peak of apoptosis. Beyond 15 h, we observed no significant difference between the wt and galectin-7−/− reactions. In summary, the apoptotic response is prematurely triggered and lasts for a longer period in the absence of galectin-7.
Figure 4.
In vivo UVB irradiation. The wt and galectin-7−/− adult female mice were depilated and irradiated with 2000 J/m2 UVB. (A) Apoptotic kinetics. Sections of unirradiated (−UV) or irradiated back skin samples were stained with hematoxylin and eosin, and the number of sunburn cells per centimeter of epidermis was determined. Three to six mice per genotype were used for each time point. Each bar represents the mean value ± SD. Statistical differences between wt and mutant animals are noted *p < 0.01. (B) Proliferative response. Sections of unirradiated (−UV) or irradiated back skin samples were labeled with anti-Ki67 Ab, and the number of positive cells per centimeter of epidermis was determined. Three to six mice per genotype were used for each time point. Each bar represents the mean value ± SD (*p < 0.01) (C) Confocal analysis of galectin-7 distribution. Representative sections of unirradiated (−UV) or irradiated wt back skin samples were stained with anti-galectin-7 Ab. White lines indicate the dermoepidermal junction. Bar, 10 μm.
Unexpectedly, changes in galectin-7 expression after UVB injury were not restricted to the apoptotic sunburn cells (Bernerd et al., 1999), but they occurred in all keratinocytes of the exposed area (Figure 4C). Initially, at 6 h post-UVB, the cytoplasm of all basal and suprabasal cells was positive for galectin-7. At 12 h post-UVB, the time of maximal apoptotic response, some basal cell nuclei displayed intense galectin-7 staining. These are likely to be sunburn cells since their nuclei were picnotic (data not shown), and some of them were clearly detaching from their neighboring cells. At 18 h post-UVB, galectin-7–positive dots were present in the cytoplasm of all epidermal cells, before reappearance of the characteristic punctiform distribution of galectin-7 along the membranes at 24 h post-UVB. In conclusion, there is a clear redistribution of galectin-7 in all cells of the irradiated region of the epidermis.
Regenerative Response.
We found that the phase of cell proliferation was also severely affected in the mutant mice (Figure 4B). At 24 h post-UVB injury, the number of dividing cells in galectin-7−/− epidermis was already slightly higher than that in wt epidermis. At 48 h, there was an abrupt increase in the number of Ki67-positive cells in galectin-7−/− epidermis, resulting in 2.5-fold excess relative to wt epidermis. This hyperproliferative activity was transient since comparable numbers of Ki67-positive cells were detected at 72 and 96 h in both mutant and wt epidermis. In summary, there is a burst of proliferation taking place in the galectin-7−/− epidermis, whereas during the 24- to 96-h period post-UVB, the number of dividing cells increased moderately and regularly in wt epidermis.
Defective Wound-healing Capacity of Galectin-7–deficient Mice
The extent to which galectin-7 may play a role in posttraumatic skin repair was also assessed after wounding in vivo (Figure 5). We made a superficial scratch along the length of the tail, which damaged the epidermis but left the dermis mostly intact. In these conditions, the wound was closed and restratification was well underway 48 h after injury (Figure 5A). We thus studied the dynamics of the healing process during this time frame. At 24 h, we found that the closure of the wound was less advanced in the galectin-7−/− than in the wt animals (20% difference, p < 0.05) (Figure 5B). At 48 h, closure was completed in both control and experimental groups.
Figure 5.
In vivo wound-healing experiments after tail injury. Superficial scratches were made along the sagittal axis of the tail of wt and galectin-7−/− adult mice. (A) Transverse cryosections of injured wt tails at 24 and 48 h after injury were stained with hematoxylin and eosin. Bar, 75 μm. Arrows indicate the wound margins. (B) Distance between the two wound margins 24 h after injury in wt and galectin-7−/− mice. Results were calculated as a mean of three independent measurements per animal. (C) Immunohistochemical detection of Ki67-positive nuclei at the wound site (left). Number of Ki67-positive cells at 24 h and 48 h after wounding (right). Results were calculated as a mean of three independent measurements per animal. (D and E) Double immunostaining with anti-galectin-7 Ab (red) and anti-cortactin Ab (green) of the wound margins, 24 h after injury. Nuclei were detected by Hoechst 33342 staining. Arrows indicate the direction of migration. Note the ectopic cortactin-positive domains (arrowheads) in cells away from the leading edge in galectin-7−/− explants. Bar, 10 μm.
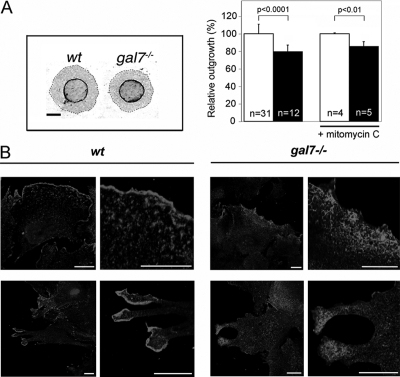
When we studied cell proliferation at 24 h after injury, we found that the number of Ki67-positive cells was similar between mutant and wt mice, indicating that the delay in wound healing was not due to a defect in proliferation, implying a defect in cell migration (Figure 5C). Accordingly, we observed that galectin-7 specifically accumulates at the front of the leading edge keratinocytes where it colocolizes with cortactin, an actin-binding protein implicated in membrane ruffle formation (Figure 5D). In the absence of galectin-7, an intense and diffuse cortactin signal is observed in keratinocytes at the wound margin (Figure 5E). Moreover, aberrant membrane accumulations of cortactin are seen in some mutant keratinocytes located behind the leading edge (see arrowheads in Figure 5E). This was repeatedly observed with several mutant animals and never occurred in wt cells. Collectively, these results argue in favor of a role for galectin-7 in keratinocyte migration during wound repair.
At 48 h after injury, when the wound is closed and the process of regeneration is taking place, we observed an intense hyperproliferative reaction in the mutant epidermis compared with the wt tissue (Figure 5C). Together with the data presented in Figure 4B, we conclude that galectin-7 plays a key role in the control of keratinocyte proliferation after both types of injury.
To further investigate the role of galectin-7 in keratinocyte migration, we used a recently established ex vivo wound-healing assay. In this assay, newborn circular skin biopsies are placed in culture and keratinocytes move out of the explant, forming a continuous sheet around the original biopsy. This process mimics the epithelialization events taking place during skin repair after wounding in vivo. In addition, the surface of keratinocyte outgrowths gives a quantitative estimate of their epithelialization potential (Mazzalupo et al., 2002). In these conditions, we found that the surface of outgrowths derived from galectin-7−/− explants was reduced, by a factor of 20%, compared with the controls (p < 0.0001) (Figure 6, A). As predicted based on the in vivo results, this difference was due to defects in the migrating capacity of galectin-7−/− keratinocytes. Indeed, when cell division was irreversibly blocked by a treatment with mitomycin C at day 2 of culture, we still observed that mutant outgrowths were 15% smaller than wt outgrowths at day 7 (p < 0.01) (Figure 6A). In addition, we also observed striking differences in the subcellular localization of cortactin at the edge of explants. In wt cells, a characteristic sharp, intense signal was detected along the membrane protrusions whereas, in mutant cells, the signal was diffuse indicating a defect in the cortical domain of lamellopodia (Figure 6B).
Figure 6.
Galectin-7 in reepithelialization process ex vivo. (A) Picture of wt and galectin-7−/− newborn skin explants processed for keratin17 immunostaining after 7 d in culture. Dashed lines indicate the leading edge of keratinocyte outgrowths. Bar, 2 mm. Keratinocyte outgrowth area of wt (white bars) and galectin-7−/− (black bars) explants were measured either after 7 d in normal culture medium (bars on the left) or when a 2 h mitomycin-C treatment had been applied on the second day. n, number of animals used. (B) Subcellular distribution of cortactin in lamellopodia of migrating keratinocytes in wt and galectin-7−/− explants. On day 7, cultures were fixed, permeabilized and stained with anti-cortactin antibody. Nuclei were detected by Hoechst 33342 staining. Bar, 20 μm.
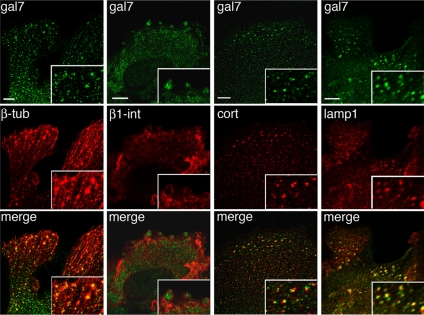
Interestingly, when we studied the distribution of galectin-7 in these cell protrusions, we detected small intracellular galectin-7–positive dots, which were particularly abundant in the protrusions of cells at the front of migration (Figure 7). These dots were located along the microtubule cables. In some cells, we also noticed the presence of larger dots or patches, in which galectin-7 and tubulin colocalized, at the extremities of the cables (Figure 7). Interestingly, these galectin-7–positive patches were surrounded by a ring of β1-integrin (Figure 7), suggesting that they corresponded to the adhesion zones called podosomes, which mediate cell/extracellular matrix (ECM) or substratum interactions. Cortactin is also known to be recruited in the core region of podosomes. The colocalization of galectin-7 and cortactin in these patches confirmed that they were podosomes (Figure 7). Finally, we observed that some of these structures were Lamp-1 positive (Figure 7), which is consistent with the recent finding that podosomes might be sites of lysosomal secretion of matrix proteases (Linder, 2007). Thus, galectin-7 is recruited in podosomes, which may account for its role in keratinocyte migration.
Figure 7.
Confocal analysis of galectin-7 distribution in leading edge keratinocytes. Double immunostainings with anti-galectin-7 Ab combined with anti-β-tubulin, anti-β1-integrin, anti-cortactin, or anti-Lamp1 Abs were performed on wt skin explants fixed after 7 d in culture. Pictures were taken in the focal plane of cell adhesion structures. Bar, 10 μm.
DISCUSSION
We have generated galectin-7–deficient mice which seem normal, and do not display overt phenotypes regarding survival or fertility. These findings suggest that galectin-7 may be a dispensable protein for all vital functions important in animal house conditions. Because galectin-7 is strongly expressed in keratinocytes, we focused our study on the role of this protein in epidermis. We could not find any difference in adult epidermal tissue between galectin-7 null mice and their wt littermates, neither by ultrastructural analysis nor by immunostaining using a large panel of markers. Adult skin structure, organization, and differentiation seemed to be normal despite the absence of this major component of keratinocytes. Although some subtle developmental abnormalities cannot be excluded, these results rule out any critical role for galectin-7 in skin morphogenesis, notably in the process of stratification, as we had suggested previously (Timmons et al., 1999).
In contrast, we did find that the lack of galectin-7 disturbed the epidermal response to environmental injury. After UVB exposure in vivo, the time course of the apoptotic response was affected in the null mutant epidermis; sunburn cells occurred earlier, the response was less acute, and lasted longer compared with wt tissue. These results are not what might be expected from a mutation in a proapoptotic gene, which galectin-7 was reported to be (Bernerd et al., 1999; Kuwabara et al., 2002). Rather, galectin-7 operates in vivo as a fine tuner, ensuring the completion of a robust and short apoptotic reaction in response to UVB stress. It is worth noting that this information could only have been obtained by examining the kinetics of the apoptotic response over the entire period of 24 h. A single time point, as is commonly reported in the field (Ziegler et al., 1994; Gillardon et al., 1999; Grossman et al., 2001), would have been misleading. For example, at 12 h post-UVB, the reduced number of apoptotic cells in the mutant mice could have been taken as a proof of a proapoptotic role, whereas the opposite conclusion could have been drawn from the results obtained at 9 h post-UVB.
The extent to which galectin-7 may play a role in posttraumatic skin response was also assessed in the context of mechanical injury in vivo. We found that the process of wound closure was less efficient in the absence of galectin-7 (20% wider wound bed in mutant skin compared with wt at 24 h after injury). In an ex vivo assay for wound healing, keratinocyte outgrowth from galectin-7−/− skin explants was also reduced by 20% compared with the wt controls. Because this difference was maintained when proliferation was blocked by mitomycin-C treatment, we conclude that it is primarily due to reduced keratinocyte migration. Consistently, cortactin distribution is severely affected in migrating keratinocytes lacking galectin-7, suggesting that the formation and/or stabilization of actin-based lamellopodia is abnormal. In addition, the coordination of events implicated in wound closure seems to be perturbed in the absence of galectin-7 because cortactin is ectopically expressed in cells located away from the front. How are these effects mediated by galectin-7 remains to be elucidated, but, one clue came from the study of its fine distribution in migrating cells. Hence, we observed that galectin-7 accumulates in podosomes, which are specialized cell–matrix adhesion complexes connecting the ECM to the microfilament network (Spinardi et al., 2004). We propose therefore that galectin-7 might participate in keratinocyte migration by modulating the stability of these highly flexible and dynamic structures. Alternatively, galectin-7 may be secreted through the posodomes, which would be consistent with the recent discovery that these structures are sites of secretion (Linder, 2007). In support of this hypothesis, Cao et al. (2002) found that adding exogenous galectin-7 accelerates the rate of healing of wounded cornea. Once externalized, like other members of this gene family, galectin-7 might function as a matricellular molecule modulating adhesion, for example by regulating interactions between integrins and ECM components and/or by rapidly remodelling local ECM (Hikita et al., 2000; Levy et al., 2001; Elola et al., 2007).
Surprisingly, we also detected a significant increase in keratinocyte proliferation in galectin-7 mutants after both types of injury. This intense proliferation occurred during the regeneration of the damaged tissue, i.e., after completion of the post-UVB apoptotic response and after wound closure. Because the level of cell death is comparable between wt and mutant epidermis after UVB damage, the excess proliferation observed in the mutant is likely to be a primary consequence of the galectin-7 mutation, and not a secondary response to an excessive loss of cells. These results reveal therefore a potential function for galectin-7 in regulating keratinocyte proliferation during tissue repair. Although there is precedent for other galectins having roles in cell growth and cell cycle progression (Lin et al., 2002; Fischer et al., 2005), this is the first report showing the importance of galectin-7 in cell proliferation. The hyperproliferation documented here in the absence of galectin-7 was triggered by only a single dose of UVB, or a mild mechanical injury. It will be interesting to assess the consequences of subjecting galectin-7−/− skin to further stress such as chronic UVB irradiation. Such a regimen might lead to various skin disorders ranging from benign skin hyperplasia to increased incidence of cancer.
In conclusion, these in vivo experiments provide an integrated and dynamic picture of the multiple roles of galectin-7 at the level of the entire tissue. We now have genetic evidence that galectin-7 plays a critical role in the maintenance of epidermal homeostasis by modulating keratinocyte apoptosis and proliferation as well as participating in the process of cell migration. Because galectin-7 is an abundant component of normal keratinocytes, it is possible that it is present as a “sentinelle” molecule, constantly sensing the integrity of the tissue in steady-state conditions while contributing to posttraumatic skin regeneration under environmental stress.
ACKNOWLEDGMENTS
We are grateful to Danielle Bucchini for blastocyst injections and to Pierrette Desbois who contributed to the initial part of this work. We also thank the members of the Institut Jacques Monod Animal Facility, Imaging and Electron microscopy services. We are grateful to Pierre Coulombe, Gabriel Rabinovich, Kurt Drickamer, Véronique Proux, Roger Karess, and Jérôme Collignon for support. G. G. was a recipient of Ministère de l'Education Nationale, de la Recherche et de la Technologie and Association pour la Recherche sur le Cancer (ARC) fellowships. This work was supported by Groupement d'Entreprises Françaises duns la Lutte contre le Cancer, Ligue Contre le Cancer (comité de Paris), and ARC (4680) grants allocated to F. P and by ARC grant 9500, Fondation de l'Avenir, Société Française de Dermatologie, and Association Francaise contre les Myopathies grants allocated to T. M.
Abbreviations used:
- Ab
antibody
- ECM
extracellular matrix
- CRD
carbohydrate recognition domain
- UV
ultraviolet
- wt
wild type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-02-0166) on October 1, 2008.
REFERENCES
- Barondes S. H., et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994a;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994b;269:20807–20810. [PubMed] [Google Scholar]
- Bernerd F., Sarasin A., Magnaldo T. Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. Proc. Natl. Acad. Sci. USA. 1999;96:11329–11334. doi: 10.1073/pnas.96.20.11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernot K. M., Coulombe P. A., Wong P. Skin: an ideal model system to study keratin genes and proteins. Methods Cell Biol. 2004;78:453–487. doi: 10.1016/s0091-679x(04)78016-4. [DOI] [PubMed] [Google Scholar]
- Blois S. M., et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Cao Z., Said N., Amin S., Wu H. K., Bruce A., Garate M., Hsu D. K., Kuwabara I., Liu F. T., Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J. Biol. Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- Cao Z., Said N., Wu H. K., Kuwabara I., Liu F. T., Panjwani N. Galectin-7 as a potential mediator of corneal epithelial cell migration. Arch. Ophthalmol. 2003;121:82–86. doi: 10.1001/archopht.121.1.82. [DOI] [PubMed] [Google Scholar]
- Delacour D., Cramm-Behrens C. I., Drobecq H., Le Bivic A., Naim H. Y., Jacob R. Requirement for galectin-3 in apical protein sorting. Curr. Biol. 2006;16:408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Elola M. T., Wolfenstein-Todel C., Troncoso M. F., Vasta G. R., Rabinovich G. A. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol. Life Sci. 2007;64:1679–1700. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C., Sanchez-Ruderisch H., Welzel M., Wiedenmann B., Sakai T., Andre S., Gabius H. J., Khachigian L., Detjen K. M., Rosewicz S. Galectin-1 interacts with the {alpha}5{beta}1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 2005;280:37266–37277. doi: 10.1074/jbc.M411580200. [DOI] [PubMed] [Google Scholar]
- Gillardon F., Moll I., Meyer M., Michaelidis T. M. Alterations in cell death and cell cycle progression in the UV-irradiated epidermis of bcl-2-deficient mice. Cell Death Differ. 1999;6:55–60. doi: 10.1038/sj.cdd.4400455. [DOI] [PubMed] [Google Scholar]
- Grossman D., Kim P. J., Blanc-Brude O. P., Brash D. E., Tognin S., Marchisio P. C., Altieri D. C. Transgenic expression of survivin in keratinocytes counteracts UVB-induced apoptosis and cooperates with loss of p53. J. Clin. Invest. 2001;108:991–999. doi: 10.1172/JCI13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita C., Vijayakumar S., Takito J., Erdjument-Bromage H., Tempst P., Al-Awqati Q. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J. Cell Biol. 2000;151:1235–1246. doi: 10.1083/jcb.151.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi J., Kasai K. The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- Hsu D. K., Liu F. T. Regulation of cellular homeostasis by galectins. Glycoconj. J. 2004;19:507–515. doi: 10.1023/B:GLYC.0000014080.95829.52. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/s0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- Ioffe E., Liu Y., Bhaumik M., Poirier F., Factor S. M., Stanley P. WW 6, an embryonic stem cell line with an inert genetic marker that can be traced in chimeras. Proc. Natl. Acad. Sci. USA. 1995;92:7357–7361. doi: 10.1073/pnas.92.16.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara I., Kuwabara Y., Yang R. Y., Schuler M., Green D. R., Zuraw B. L., Hsu D. K., Liu F. T. Galectin-7 (PIG1) exhibits pro-apoptotic function through JNK activation and mitochondrial cytochrome c release. J. Biol. Chem. 2002;277:3487–3497. doi: 10.1074/jbc.M109360200. [DOI] [PubMed] [Google Scholar]
- Lahm H., Andre S., Hoeflich A., Kaltner H., Siebert H. C., Sordat B., von der Lieth C. W., Wolf E., Gabius H. J. Tumor galectinology: insights into the complex network of a family of endogenous lectins. Glycoconj. J. 2004;20:227–238. doi: 10.1023/B:GLYC.0000025817.24297.17. [DOI] [PubMed] [Google Scholar]
- Leonidas D. D., Vatzaki E. H., Vorum H., Celis J. E., Madsen P., Acharya K. R. Structural basis for the recognition of carbohydrates by human galectin-7. Biochemistry. 1998;37:13930–13940. doi: 10.1021/bi981056x. [DOI] [PubMed] [Google Scholar]
- Levy Y., Arbel-Goren R., Hadari Y. R., Eshhar S., Ronen D., Elhanany E., Geiger B., Zick Y. Galectin-8 functions as a matricellular modulator of cell adhesion. J. Biol. Chem. 2001;276:31285–31295. doi: 10.1074/jbc.M100340200. [DOI] [PubMed] [Google Scholar]
- Lin H. M., Pestell R. G., Raz A., Kim H. R. Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene. 2002;21:8001–8010. doi: 10.1038/sj.onc.1205820. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Lindstedt R., Apodaca G., Barondes S. H., Mostov K. E., Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J. Biol. Chem. 1993;268:11750–11757. [PubMed] [Google Scholar]
- Liu F., Patterson R., Wang J. Intracellular functions of galectins. Biochim. Biophys. Acta. 2002;1572:263. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Rabinovich G. A. Galectins as modulators of tumour progression. Nat. Rev. Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Madsen P., Rasmussen H. H., Flint T., Gromov P., Kruse T. A., Honore B., Vorum H., Celis J. E. Cloning, expression, and chromosome mapping of human galectin-7. J. Biol. Chem. 1995;270:5823–5829. doi: 10.1074/jbc.270.11.5823. [DOI] [PubMed] [Google Scholar]
- Magnaldo T., Bernerd F., Darmon M. Galectin-7, a human 14-kDa S-lectin, specifically expressed in keratinocytes and sensitive to retinoic acid. Dev. Biol. 1995;168:259–271. doi: 10.1006/dbio.1995.1078. [DOI] [PubMed] [Google Scholar]
- Magnaldo T., Fowlis D., Darmon M. Galectin-7, a marker of all types of stratified epithelia. Differentiation. 1998;63:159–168. doi: 10.1046/j.1432-0436.1998.6330159.x. [DOI] [PubMed] [Google Scholar]
- Mazzalupo S., Wawersik M. J., Coulombe P. A. An ex vivo assay to assess the potential of skin keratinocytes for wound epithelialization. J. Invest. Dermatol. 2002;118:866–870. doi: 10.1046/j.1523-1747.2002.01736.x. [DOI] [PubMed] [Google Scholar]
- Plachta N., Annaheim C., Bissiere S., Lin S., Ruegg M., Hoving S., Muller D., Poirier F., Bibel M., Barde Y. A. Identification of a lectin causing the degeneration of neuronal processes using engineered embryonic stem cells. Nat. Neurosci. 2007;10:712–719. doi: 10.1038/nn1897. [DOI] [PubMed] [Google Scholar]
- Poirier F. Roles of galectins in vivo. Biochem. Soc. Symp. 2002;69:95–103. doi: 10.1042/bss0690095. [DOI] [PubMed] [Google Scholar]
- Polyak K., Xia Y., Zweier J. L., Kinzler K. W., Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A., Liu F. T., Hirashima M., Anderson A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 2007;66:143–158. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Sato S., Burdett I., Hughes R. C. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp. Cell Res. 1993;207:8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- Sato S., Nieminen J. Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj. J. 2004;19:583–591. doi: 10.1023/B:GLYC.0000014089.17121.cc. [DOI] [PubMed] [Google Scholar]
- Saussez S., Kiss R. Galectin-7. Cell Mol. Life Sci. 2006;63:686–697. doi: 10.1007/s00018-005-5458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L., Rietdorf J., Nitsch L., Bono M., Tacchetti C., Way M., Marchisio P. C. A dynamic podosome-like structure of epithelial cells. Exp. Cell Res. 2004;295:360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Takenaka Y., Fukumori T., Raz A. Galectin-3 and metastasis. Glycoconj. J. 2004;19:543–549. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- Thijssen V. L., Poirier F., Baum L. G., Griffioen A. W. Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood. 2007;110:2819–2827. doi: 10.1182/blood-2007-03-077792. [DOI] [PubMed] [Google Scholar]
- Timmons P. M., Colnot C., Cail I., Poirier F., Magnaldo T. Expression of galectin-7 during epithelial development coincides with the onset of stratification. Int. J. Dev. Biol. 1999;43:229–235. [PubMed] [Google Scholar]
- Toscano M. A., et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Jonason A. S., Leffell D. J., Simon J. A., Sharma H. W., Kimmelman J., Remington L., Jacks T., Brash D. E. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]