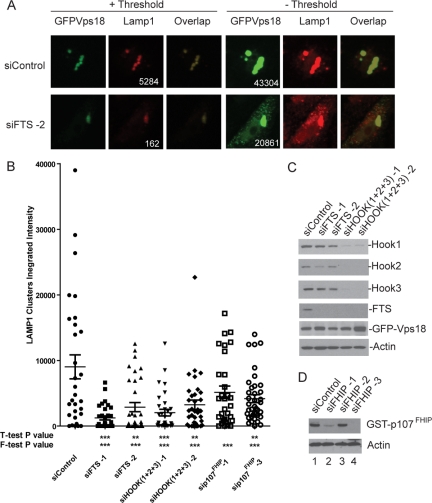
Figure 6.
Depletion of FHF complex components reduces the ability of Vps18 to promote late endosome/lysosome clustering. (A) HeLa cells were transfected with control siRNA or siRNA targeting FTS. After 48 h, cells were transfected with a plasmid expressing GFP-Vps18, and 60 h later, late endosomal/lysosomal clusters were examined by immunofluorescence using anti-LAMP1 antibodies in conjunction with detection with Alexa598-conjugated secondary antibodies (red). GFP-Vps18 was identified by GFP fluorescence (green). To determine the integrated intensity for LAMP1 within clusters, a threshold (+ Threshold) was applied such that the maximal pixel signal was in the linear range. In the absence of threshold (− Threshold), individual vesicles not present within clusters can be seen in cells wherein FTS was depleted. Integrated intensities of Vps18 before thresholding (including both clustered Vps18 and dispersed Vps18), as well as LAMP1 aggregates after thresholding, are presented. The integrated intensities for LAMP1 and GFP-Vps18 are shown. The integrated intensities for GFP-Vps18 ranged from ∼20,000 to ∼40,000 over all the cells analyzed. (B) Quantification of the effects of FTS depletion on Vps18-mediated endosomal clustering. The integrated intensity of LAMP1 within GFP–Vps18-positive clusters was determined using MetaMorph software as described in Materials and Methods for 10–15 cells in each of three independent experiments (30–45 cells total). The mean ± SEM is indicated. Depletion of the indicated proteins displayed statistical significance using the F-test (***p < 0.001). (C–D) Immunoblotting of extracts from cells transfected with the indicated siRNAs as described in A. Extracts were separated by SDS-PAGE and blots probed with the indicated antibodies. The anti-FTS antibodies used here were affinity purified. To demonstrate depletion of p107FHIP, cells were cotransfected with a vector expressing GST-p107FHIP and extracts blotted with anti-GST antibodies.