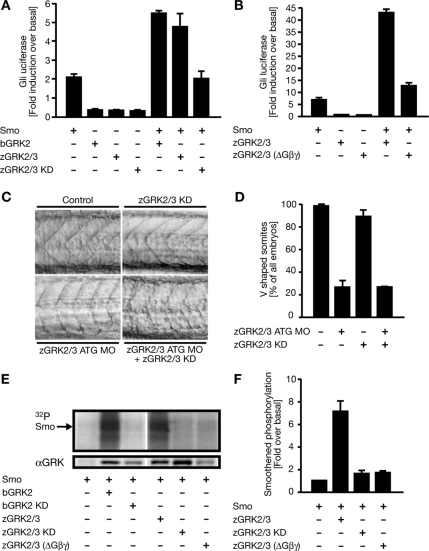
Figure 3.
zGRK2/3 stimulates Hh signaling through Smo and directly phosphorylates Smo. (A) Smo (human) signaling in C3H10T1/2 cells promoted by zGRK2/3 and the kinase-dead zGRK2/3 KD (K220R) mutant is compared with the bovine protein (bGRK2). (B) Recruitment by G proteins is indispensable for the effect of zGRK2/3 on Smo-mediated signaling. zGRK2/3 (ΔGβγ) is characterized by a point mutation (R587Q) described as necessary for interaction with the βγ-subunit of a G protein. (C) Endogenous Smo signaling depends on the kinase function of zGRK2/3 in fish, as rescue experiments using zGRK2/3 KD mRNA failed. Embryos injected with either zGRK2/3 ATG MO alone or in combination with zGRK2/3 KD mRNA still developed U-shaped somites (n = three sets of injections). (D) Graph showing percentages of embryos developing V-shaped somites under different treatments, depicted as mean ± SEM (n = 2 experiments). (E) Representative gel showing the phosphorylation of Smo by GRKs in HEK293T cells, which were transfected either with Smo alone or in combination with bovine bGRK2, bGRK2 kinase-dead (KD), zGRK2/3, or zGRK2/3 KD. White line indicates deletion of blank lanes in the gel. (F) Quantification of phosphorylation of Smo by zGRK2/3. Error bars, SD of two experiments.