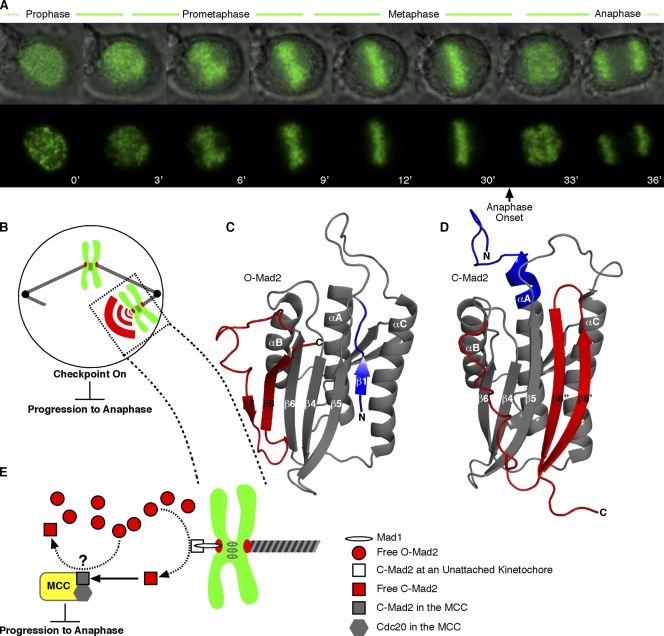
Figure 1.
The mitotic checkpoint ensures equal partitioning of chromosomes in anaphase. (A) A human tissue culture cell progressing through mitosis with time indicated in minutes. In the top row, chromosomes (green) are overlaid with a differential interference contrast image of the entire cell. Sister chromatids align at the metaphase plate early in mitosis and wait for ∼20 min before chromatid separation in anaphase. Upon final chromosome alignment, the mitotic checkpoint signal decays, allowing the cell to enter anaphase and initiate simultaneous separation of sister chromatids. (B) The mitotic checkpoint signal, comprised in part by a diffusible pool of C-Mad2, emanates from kinetochores that have not yet properly engaged the microtubule-based spindle. A single unattached chromosome is sufficient to generate a checkpoint signal that arrests mitosis before anaphase. (C and D) Interconversion between inactive O-Mad2 (PDB 1DUJ; Luo et al., 2000) and checkpoint-active C-Mad2 (PDB 1S2H; Luo et al., 2004) involves a major secondary and tertiary structural reorganization of N-terminal (blue) and C-terminal (red) segments. (E) Unattached kinetochores contain the checkpoint protein Mad1, which recruits C-Mad2, providing a catalytic surface for the conversion of the soluble pool of inactive O-Mad2 to active C-Mad2. C-Mad2 is able to bind and inhibit Cdc20 within the MCC, halting progression to anaphase. The Cdc20–C-Mad2 complex may also act to catalyze conversion of the O-Mad2 pool, although this aspect of Mad2 signaling remains controversial (Yu, 2006; Musacchio and Salmon, 2007). (B and E) Chromosomes are drawn in green with their kinetochores drawn in red.