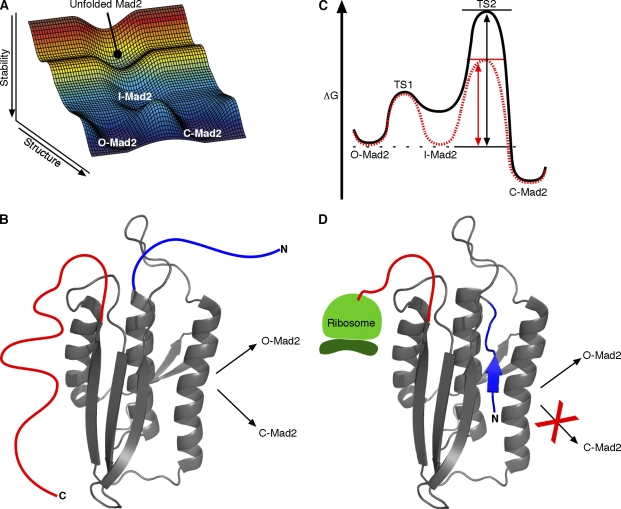
Figure 3.
Mad2 unfolding and refolding considerations. (A) Free energy reaction landscape for Mad2 interconversion through a partially folded intermediate that lies on the folding pathway (created with Matlab version R2007a; The MathWorks, Inc.). When chemically denatured Mad2 is refolded, it initially reaches a nonequilibrium O-Mad2–C-Mad2 mixture, suggesting that the folding pathway to reach either form passes through a common intermediate and kinetically partitions rather than passing through one form on the way to the other. We suggest that the catalyzed interconversion seems likely, on this and other grounds, to pass back through the same partially unfolded intermediate. (B) A notional structure for a Mad2-folding intermediate showing the common (gray) and variable (colored as in Fig. 1 C) segments. (C) Catalysis through intermediate stabilization. The conversion reaction of Mad2 is drawn with (red dashed line) or without (black solid line) dimerization with the kinetochore-bound C-Mad2–Mad1 complex. The measured C-Mad2/O-Mad2 equilibrium ratio is 8:1 (Luo et al., 2004), indicating that C-Mad2 is ∼1 kcal/mol more stable than O-Mad2. Conformational selection of I-Mad2 would equally stabilize I-Mad2 and the TS2 transition barrier relative to O-Mad2, effectively increasing the O-Mad2→C-Mad2 rate even though the energy difference between I-Mad2 and TS2 remains unchanged. The dashed black line indicates the energy state of O-Mad2, the black arrow (left) indicates increasing energy, the double-headed black arrow indicates the energy difference between O-Mad2 and TS2 without dimerization, and the doubled-headed red arrow indicates the energy difference between O-Mad2 and TS2 with dimerization. (D) As it emerges from the ribosome, Mad2 may preferentially fold to O-Mad2 because the last emerging C-terminal segment is required for forming C-Mad2, and protein folding is typically much faster than translation.