Abstract
Gene fscTE, encoding a putative type II thioesterase (TEII), was associated with the FR-008/candicidin gene cluster. Deletion of fscTE reduced approximately 90% of the FR-008/candicidin production, while the production level was well restored when fscTE was added back to the mutant in trans. FscTE was unable to compensate for the release of the maturely elongated polyketide as site-directed inactivation of the type I thioesterase (TEI) totally abolished FR-008/candicidin production. Direct biochemical analysis of FscTE in parallel with its homologue TylO from the tylosin biosynthetic pathway demonstrated their remarkable preferences for acyl-thioesters (i.e., propionyl-S-N-acetylcysteamine [SNAC] over methylmalonyl-SNAC and acetyl-SNAC over malonyl-SNAC) and thus concluded that TEII could maintain effective polyketide biosynthesis by selectively removing the nonelongatable residues bound to acyl carrier proteins. Overexpression of FscTE under the strong constitutive ermE*p promoter in the wild-type strain did not suppress FR-008/candicidin formation, which confirmed its substrate specificity in vivo. Furthermore, successful complementation of the fscTE mutant was obtained with fscTE and tylO, whereas no complementation was detected with nonribosomal peptide synthetase (NRPS) TEII tycF and srfAD, reflecting substrate specificities of TEIIs distinctive from those of either polyketide synthases or NRPSs.
Complex polyketides are a large family of bacterial natural products possessing a wide range of biological activities. The carbon frameworks of these compounds are assembled by a common mechanism of decarboxylative condensations of simple malonate derivatives (e.g., malonyl or methylmalonyl) by polyketide synthases (PKSs) in a manner very similar to fatty acid biosynthesis (14). Type I PKSs are complexes of large multimodular enzymes that catalyze biosynthesis of polyketide compounds via repetitive reaction sequences, during which each step is catalyzed by a separate enzymatic domain (7, 15, 33). The biosynthesis logic of type I PKS is also shared by nonribosomal peptide synthetases (NRPSs), which are organized into coordinated modules minimally consisting of adenylation (A), peptidyl carrier protein (PCP), and condensation (C) domains required for an elongation cycle in assembly line arrays (8). The fully extended polyketide or oligopeptide chains bound to terminal enzymatic templates as acyl-acyl carrier protein (ACP) or aminoacyl-PCP thioesters are usually released and cyclized by a type I thioesterase (TEI) domain fused to the carboxyl terminus of the last elongation module (11, 34). Although TEIs have been verified to be sufficient for chain release and cyclization by many in vitro analysis (11, 19, 32, 34), additional thioesterase genes encoding discrete proteins called type II thioesterases (TEIIs) were also found within many type I PKSs and NRPS gene clusters (3-6, 16, 20, 21, 30, 41). Sequence analysis has revealed TEIIs as belonging to the α/β-hydrolase superfamily with a catalytic triad consisting of Ser-Asp-His (21).
TEIIs are generally believed to function as an editing enzyme to restore the biosynthetic machinery by hydrolytically removing the aberrant acyl groups blocking ACP or PCP for further elongation procedures (13, 18, 31). Disruption of the TEII genes greatly reduced the productivity of several antibiotics (3, 6, 30). Coexpression of cognate TEII with PKS in heterologous hosts evidently enhanced polyketide production: e.g., the improved biosyntheses of erythromycin and picromycin aglycones in recombinant strains (27, 37). As the first biochemical evidence of TEII in type I PKS, TylO from tylosin biosynthesis hydrolyzed the acyl-N-acetylcysteamine (acyl-NAC) thioesters simulating the aberrant residues bound to ACP (13). PikAV TEII from picromycin biosynthesis was also tested for its hydrolytic activities with either elongatable or nonelongatable residues attached to ACPs (18). However, PikAV is deficiently correlated with picromycin biosynthesis efficiency as deletion of the TEII gene did not reduce product yield (5).
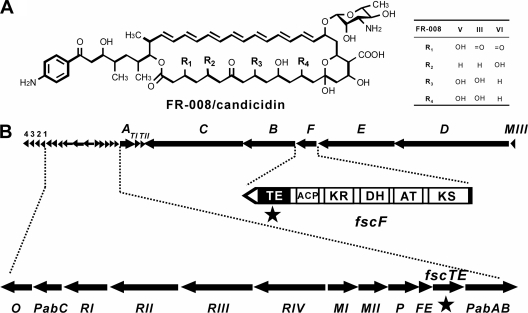
FR-008/candicidin, a heptaene macrolide antifungal agent, was synthesized by a type I PKS pathway in Streptomyces sp. strain FR-008 (Fig. 1) (4, 15, 43). One representative TEII encoded by fscTE associated with the FR-008/candicidin (fsc) gene cluster shows 48% identity, on the amino acid level, to the TEII (TylO) from the tylosin biosynthetic pathway (13). In order to elucidate the substrate specificities of TEII for its editing role in PKS, systematic analyses of FscTE and its homologue TylO were performed through in vivo gene inactivation followed by complementation and through in vitro catalysis assays with synthesized acyl-thioesters simulating the aberrant or normal extender units in polyketide elongation processes. As an extended insight into the editing role of TEII in PKS, evidence presented here strongly supports distinct substrate specificities of TEII with a capability of selectively removing the nonelongatable residues from the unprocessed PKS proteins, and thus ensuring efficient polyketide biosynthesis.
FIG. 1.
Molecular structure of FR-008/candicidin (A) and the gene cluster associated with FR-008/candicidin biosynthesis (B). A TEII encoded by fscTE and a TEI encoded by the C-terminal region of fscF are marked by stars.
MATERIALS AND METHODS
Bacterial strains, plasmids, culture conditions, and general techniques.
Streptomyces sp. strain FR-008 is the wild-type producer of FR-008/candicidins (15). Escherichia coli DH10B was used as cloning host. pBluescript II SK(+) (35), pIJ2925 (17), and PMD 18-T vector (TaKaRa) were used for plasmid constructions. pHZ1358 (36) was used for gene replacement in Streptomyces sp. strain FR-008, while pIB139 (39)—a pSET152 derivative with the ermE*p promoter (2) and a polylinker—was used for mutant complementation. SFM medium (2% agar, 2% mannitol, 2% soybean powder [pH 7.4]) was used for sporulation, fermentation, and conjugation. YEME (0.3% yeast extract, 0.5% peptone, 0.3% malt extract, 1% glucose, 0.3% sucrose [pH 7.2]) liquid medium was used for fermentation (30°C, 2 days). Trypticase soy broth supplemented with 10.3% sucrose and 1% yeast extract was used for growth of mycelia for isolation of total DNA. LB medium was used for E. coli propagation. Recombinant DNA techniques were described by Sambrook et al. (29). PCRs were performed using KOD-Plus (Toyobo) or Taq DNA polymerase.
Fermentation and analysis of polyene macrolide titer.
Polyene samples were extracted with methanol from the spores harvested from agar plates (6 days at 30°C) or the mycelia harvested from liquid fermentation (2 days at 30°C). The antibiotic titer was evaluated through high-performance liquid chromatography (HPLC) analysis at 380 nm. Component FR-008-III was used as the standard for titer comparisons because the abundance ratio of the three main FR-008/candicidin components is almost invariant in the extracts of the wild-type and recombinant strains.
Liquid chromatography-mass spectrometry (LC-MS) analysis was performed using the Agilent 1100 series LC/MSD Trap system. An Agilent Eclipse XDB-C18 column with dimensions of 4.6 by 250 mm was used, and the mobile phase was 45% CH3CN in 5.5 mM ammonium acetate (pH 4.5) at a flow rate of 0.6 ml/min. The ion trap mass spectrometer was operated with the electrospray ionization source in negative or positive mode. Drying gas flow was 10 liters/min, and nebulizer pressure was 50 lb/in2. Drying gas temperature was 350°C. The fragmentation amplitude varied between 1.0 and 1.8 V.
Plasmid construction.
Plasmid construction is described in the supplemental material.
Cloning, expression, and purification of FscTE and TylO.
A 929-bp NdeI-EcoRI DNA fragment containing fscTE was excised from pJTU598 and cloned into the expression vector pET28a digested with NdeI and EcoRI to generate pJTU2224. pJTU2224 was transformed into E. coli BL21(DE3)/pLysE for induced expression of FscTE. pMLH27 (13) was also transformed into E. coli BL21(DE3)/pLysE for expression of the previously described TylO. A 1-liter culture of each recombinant strain was grown using LB medium containing kanamycin (25 mg/ml) and chloramphenicol (12.5 mg/ml). The expression of the N-terminal His6-tagged recombinant proteins was induced at an optical density at 600 nm of 0.8 with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and incubation was continued for 20 h at 18°C.
The cells from each 1-liter culture were harvested by centrifugation at 5,000 × g for 10 min, resuspended in 25 ml of buffer A (50 mM Tris, 300 mM NaCl [pH 7.4]), and lysed by ultrasonication with a Sanyo Soniprep 150. Cell debris was removed by ultracentrifugation (twice for 30 min each at 15,000 × g), and the supernatant was loaded onto a 5-ml Hitrap chelating HP column (Amersham Biosciences). Protein purification procedures were performed at 4°C with an ÄKTA fast protein liquid chromatograph. The flow rate was 0.5 ml/min, and the absorbance was monitored at 280 nm. The column was washed with buffer A followed by a linear gradient of buffer B (50 mM Tris, 300 mM NaCl, 500 mM imidazole [pH 7.4]): from 0 to 100% buffer B over 20 min and 100% buffer B for 5 min. For the activity assay, the protein was concentrated and exchanged into 100 mM phosphate buffer (pH 7.4) with the Amicon Ultra-15 (Millipore) and mixed with 20% glycerol for storage at −80°C. Protein concentrations were determined by Bradford assay (Bio-Rad). Typically, 1 liter of culture yielded 8.8 mg of FscTE and 6.2 mg of TylO.
Hydrolytic activity assay of TEII.
Hydrolysis of acyl-S-N-acetylcysteamine (acyl-SNAC) thioesters releases thiol, which reacts with 5,5′-dithio-2-nitrobenzoic acid (DTNB) to form the detectable chromophore 5-thio-2-nitrobenzoate (λmax, 412 nm; ɛ, 13,600/M/cm) by spectrophotometer (PerkinElmer Lambda 650). To establish the time course of the TEII-catalyzed hydrolysis at 30°C, each assay mixture contained (in a total of 700 μl) 100 mM sodium phosphate (pH 7.4), 1.24 μM FscTE or 1.98 μM TylO, 7 μl of 20 mM DTNB in 100 mM sodium phosphate (pH 7.4), and various amounts of substrates dissolved in dimethyl sulfoxide. Hydrolysis rates were measured over the concentration range of 10 to 70 mM for NAC thioesters. p-Aminobenzoyl-SNAC was assayed only at 5 mM. Reactions were carried out in duplicate, and the hydrolysis rates were calculated from the initial linear portion of the curves. In all cases, the rates were calibrated with the background hydrolysis without added enzyme.
Compound synthesis.
Reagents for assays and for chemical synthesis were purchased from Sigma-Aldrich Chemical Co., Ltd. The details of the procedures for synthesis of NAC thioester derivatives are described in the supplemental material. The prepared compounds were further separated on a silica gel column impregnated with copper sulfate (10) or by preparative reverse-phase HPLC (Shimadzu) on a Shimadzu C18 20- by 250-mm column at a flow rate of 5 ml/min using the mobile phase of 35% CH3CN-H2O. Nuclear magnetic resonance spectra for protons (1H NMR) were recorded on a Varian Mercury 400-MHz spectrometer.
RESULTS
Inactivation of fscTE encoding TEII drastically reduced FR-008/candicidin production.
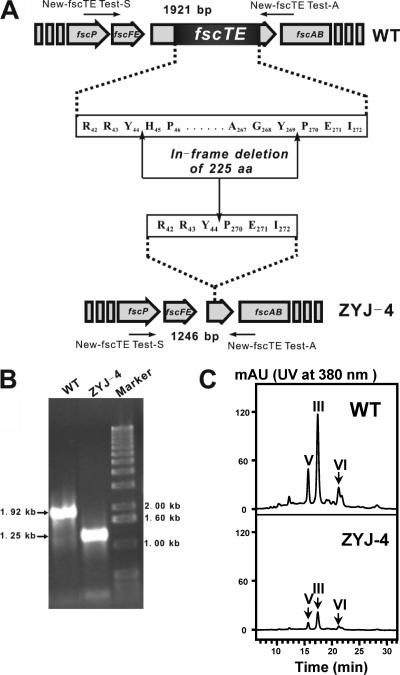
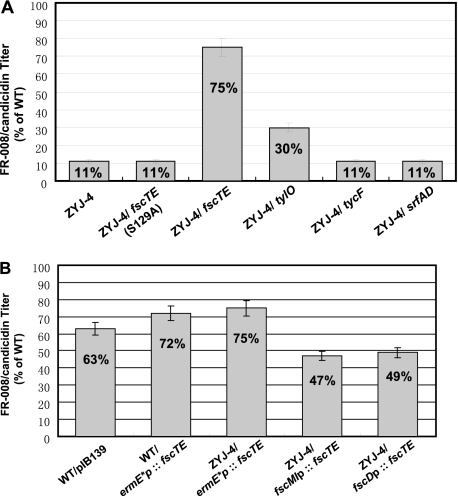
To assess the role of fscTE in FR-008/candicidin biosynthesis, a 675-bp internal DNA fragment coding for 225 amino acids (aa) of FscTE (285 aa) was in-frame deleted, which contains the entire catalytic triad assumed to be essential for thioester hydrolysis (Fig. 2A). The conjugation plasmid pJTU585, carrying a fragment with the 675-bp deletion, was introduced into Streptomyces sp. strain FR-008 by conjugation. After initial selection for thiostrepton resistance (Thior) exconjugants followed by two rounds of growth in the absence of thiostrepton, 1 Thios derivative out of 20 was selected as a desired mutant, named ZYJ-4, by PCR screening with the primers New-fscTE Test-S (5′-TCGGGCGTCCTGCTGCTCCTGCTG-3′) and New-fscTE Test-A (5′-TCGTCGTTGCGGATGACCTCGGG-3′) (Fig. 2B). ZYJ-4 was finally confirmed by sequencing the PCR product amplified from it. About 10% of the original FR-008/candicidin production of the wild-type strain could be detected in ZYJ-4 by LC-MS analysis (Fig. 2C). The necessity of FscTE for the effective FR-008/candicidin biosynthesis was further supported by the evidence that the antibiotic titer was well restored (75%) after complementation of the fscTE mutant in trans (Fig. 3A). This was achieved by placing the PCR-amplified fscTE gene under the direct control of the strong constitutive ermE*p promoter on an integrative vector of pJTU598, which was introduced into ZYJ-4 by conjugation. Furthermore, pJTU2216, as a control that differed from pJTU598 with a mutated fscTE (S129A), was also introduced into ZYJ-4 and provided no contribution to the restoration of FR-008/candicidin titer (Fig. 3A).
FIG. 2.
Inactivation of TEII gene fscTE in Streptomyces sp. strain FR-008. (A) Schematic in-frame deletion of 225 aa in FscTE to generate fscTE mutant ZYJ-4. The expected PCR product from the wild-type (WT) strain is 1,921 bp, and that from ZYJ-4 is 1,246 bp with the primers New-fscTE Test-S and -A. (B) PCR analysis of wild-type and mutant genomic DNAs. PCR products were run on an agarose gel. (C) HPLC analysis of FR-008/candicidin production in wild-type and ZYJ-4. FR-008-V, -III, and -VI are represented by V, III, and VI, respectively. mAU, milliabsorbance units.
FIG. 3.
(A) FR-008 productions in fscTE mutant (ZYJ-4) complemented with different TEIIs. fscTE (S129A) represents site-directed mutated TEII; tylO is a PKS TEII from the tylosin biosynthetic gene cluster; tycF and srfAD represent the NRPS TEIIs, respectively, from surfactin and tyrocidine biosynthetic gene clusters. All of the TEII genes were controlled by the ermE*p promoter on the integrative vector pIB139. WT, wild type. (B) Comparison of the FR-008/candicidin productivity with various levels of fscTE expression. fscDp is a promoter controlling the PKS gene fscD. fscMIp is a promoter of the post-PKS modification gene fscMI, which is probably cotranscribed with fscTE in one operon.
Comparison of FR-008/candicidin productivity with various levels of fscTE expression.
In order to test whether overexpression of FscTE suppresses FR-008/candicidin biosynthesis by overactive cleavage of the correct residues (e.g., malonyl group) from ACPs (18), we decided to assess FR-008/candicidin biosynthesis efficiency by changing the expression level of fscTE. fscTE was respectively placed and controlled under the promoters of PKS gene fscD, a post-PKS modification gene fscMI probably cotranscribed with fscTE, or a strong constitutive ermE*p promoter. These constructs were individually introduced into the fscTE mutant and the wild type as well. In the fscTE mutant, ermE*p-controlled expression of fscTE restored FR-008/candicidin titer to 75% of the wild-type titer, much higher than that controlled by fscMIp or fscDp (47% and 49%, respectively). Noticeably, an extra copy of fscTE controlled by ermE*p did not suppress the FR-008/candicidin titer (72%) compared with that in the wild type carrying the vector pIB139 alone (63%). However, for unknown reasons, the integration of pIB139 into the chromosome of Streptomyces sp. strain FR-008 caused 37% reduced FR-008/candicidin production (Fig. 3B).
Restoration of FR-008/candicidin production in the fscTE mutant by introduction of different TEIIs.
To investigate whether FscTE is catalytically comparable to its homologs from other PKSs and NRPSs, tylO (13) was selected to represent the TEII from PKSs and tycF and srfAD (40) were selected as the representative TEIIs from NRPSs. The TEII genes were each placed under the control of the constitutive promoter ermE*p and then introduced into ZYJ-4. LC-MS analysis of the recombinant strains indicated that tylO partially restored the FR-008/candicidin titer to 30%, much lower than the complementation effect of fscTE. Moreover, tycF and srfAD from NRPS biosynthetic gene clusters did not make any contribution to the titer restoration (Fig. 3A).
Inactivation of TEI totally destroyed FR-008/candicidin biosynthesis.
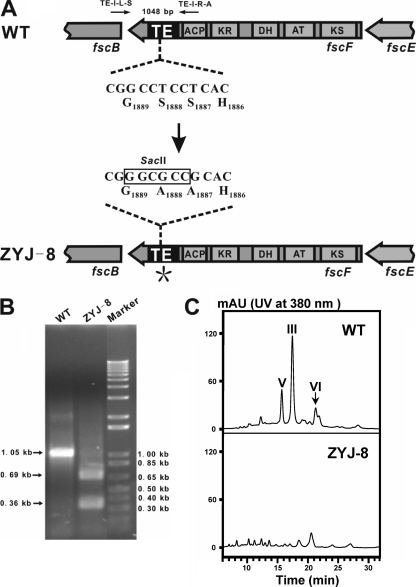
Although the release of maturely assembled polyketide is generally believed to be sufficiently carried out by TEI (11, 19, 32, 34), whether TEIIs contribute to this process is not well investigated by in vivo analysis. The active site of the TEI domain in FscF was site-specifically mutated to assess whether FR-008/candicidin was still produced without the catalysis of TEI and, therefore, to explore whether other cognate activity, e.g., TEII, could substitute TEI for the role of terminal release of FR-008/candicidin aglycone. One representative active site triad, comprised of Asp-1845, His-1941, and Ser-1887, was found in the TEI domain at the C terminus of FscF. The Ser residue in the catalytic triad was proved to be essential for the covalent attachment of the maturely assembled chain by structural elucidation and site-directed mutagenesis analysis of TEI (23, 38). For inactivation of TEI in FR-008 strain, the putative active site Ser-1887 and its neighboring residue, Ser-1888, were both changed into Ala residues and one SacII restriction site was simultaneously introduced to facilitate mutant screening (Fig. 4A). Plasmid pJTU2222, carrying the mutations S1887A and S1888A in fscF, was introduced into Streptomyces sp. strain FR-008 through conjugation. After initial selection for Thior exconjugants followed by two rounds of growth in the absence of thiostrepton, The Thios derivatives were screened by a PCR with the primers TE-I-L-S and TE-I-R-A. The desired mutant, named ZYJ-8, was selected by SacII digestion of the PCR products amplified from genomic DNA (Fig. 4B). ZYJ-8 was further confirmed through sequencing of the PCR product. The fermentation extract of ZYJ-8 was analyzed by LC-MS, and no FR-008/candicidin component was detected (Fig. 4C). The result was confirmed by analysis of three parallel mutants. Interestingly, trace amounts of polyene compounds with typical heptaene UV absorption spectra were detected in ZYJ-8, and the one with highest yield and the retention time at 21 min among them was indentified with MS spectra of [M-H]− = 754.2.
FIG. 4.
Site-directed mutation of the TEI domain in FscF. (A) Schematic mutagenesis of the TEI domain in FscF to generate mutant ZYJ-8. The putatively essential serines in the catalytic triad were changed to alanines (S1887A and S1888A). One SacII restriction site was introduced to facilitate mutant screening. WT, wild type. (B) SacII digestion of PCR products from the wild-type and mutant strains with the primers TE-I-L-S and TE-I-R-A. (C) HPLC analysis of FR-008/candicidin production in the wild type and ZYJ-8. V, III, and VI disappeared in ZYJ-8. mAU, milliabsorbance units.
Overexpression of FscTE and TylO.
FscTE and TylO were, respectively, expressed from plasmids pJTU2224 and pMLH27 (13) in E. coli as N-terminal His6-tagged recombinant proteins and purified to homogeneity by Ni2+ affinity column chromatography. The purified FscTE and TylO proteins migrated as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis with molecular masses of 35 kDa and 30 kDa (see Fig. S1 in the supplemental material), consistent with their calculated molecular masses of 33,855 Da and 29,848 Da, respectively.
Substrate specificity of FscTE and TylO.
The NAC thioesters were usually used as the substrates in thioesterase hydrolysis assays (11-13, 22, 40). In this study, acetyl-SNAC and propionyl-SNAC were prepared to mimic the aberrant acyl-ACP substrates, whereas malonyl-SNAC and methylmalonyl-SNAC were used to represent the correct substrates in the PKS condensing reactions of FR-008/candicidin and tylosin biosynthesis. On the other hand, p-aminobenzoyl-SNAC was also prepared to imitate the p-aminobenzoyl-ACP substrate in initiation of FR-008/candicidin biosynthesis (see Table S1 in the supplemental material).
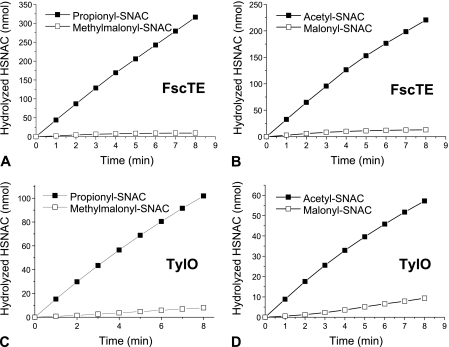
To investigate the substrate recognition of TEII, FscTE and TylO were incubated with the NAC thioester substrates. Both of FscTE and TylO showed significant efficiency for hydrolysis of acetyl-SNAC and propionyl-SNAC thioesters (Table 1), whereas very limited hydrolysis was detected with malonyl-SNAC and methylmalonyl-SNAC thioesters (Fig. 5), which excluded the practical possibility of kinetic measurement. Series of time course experiments with substrate concentration ranges of 10 to 70 mM were carried out with consistent substrate preference as presented in Fig. 5. Moreover, p-aminobenzoyl-SNAC was not identified as the proper substrate for FscTE and TylO, which suggests TEII may not interfere the initiation of FR-008/candicidin biosynthesis (data not shown).
TABLE 1.
Kinetic parameters of TEII-catalyzed NAC thioester hydrolysis
| Substrate | TEII | kcat (min−1) | Km (mM) | kcat/Km (M−1 s−1) |
|---|---|---|---|---|
| Acetyl-SNAC | FscTE | 71.6 | 33.4 | 35.7 |
| TylO | 14.8 | 42.8 | 5.8 | |
| Propionyl-SNAC | FscTE | 109.2 | 32.0 | 56.9 |
| TylO | 23.2 | 35.6 | 10.9 |
FIG. 5.
Hydrolytic activities of FscTE and TylO. (A) FscTE with propionyl-SNAC and methylmalonyl-SNAC. (B) FscTE with acetyl-SNAC and malonyl-SNAC. (C) TylO with propionyl-SNAC and methylmalonyl-SNAC. (D) TylO with acetyl-SNAC and malonyl-SNAC. The substrate concentration was 20 mM in each assay.
DISCUSSION
Some nonelongatable acyl groups, e.g., acetyl or propionyl, were inevitably generated during polyketide biosynthesis. In the absence of a suitable intermediate acyl thioester bound to the active site thiol, the ketosynthase domain can erroneously decarboxylate the chain extender units such as malonyl or methylmalonyl, resulting in acyl residues blocking ACP from further polyketide elongation procedure (13, 18). Altenatively, phosphopantetheinyl transferases (PPTases) usually utilize coenzyme A-SH as a 4′-phosphopantetheine donor for activation of apo-ACP/or PCP to the active holo form. However, PPTases occasionally can transfer acyl-phosphopantetheinyl from acyl coenzyme A to ACP/or PCP, generating the unprocessed acyl-ACP/or PCP (28, 31). Reasonably, removal of the nonelongatable acyl groups from ACP through hydrolysis by a thioesterase (e.g., TEII) is necessary to restore PKS function and maximize polyketide production.
Deletion of TEII-encoding genes generally led to a drastic reduction in product yields (3, 6, 9, 30). Consistently, in-frame deletion of the TEII-encoding gene fscTE reduced about 90% of FR-008/candicidin production, and then the titer was well restored after complementation of the mutant in trans. Additionally, complementation with site-specific mutated FscTE (S129A) was unable to restore the production, which further confirmed the catalytic activity of TEII is essential for the effective biosynthesis of FR-008/candicidin. Moreover, the fact that there were no FR-008/candicidin components detected in the TEI mutant implied that FscTE cannot substitute for the cognate TEI of the last module for polyketide termination and cyclization.
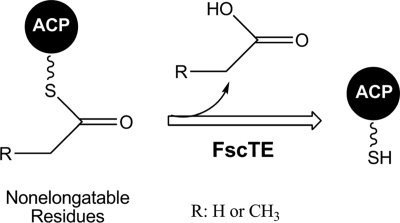
By selectively removing the nonelongatable residues blocking the ACP from further polyketide elongation, TEII likely catalyzes the regeneration of ACP domains and thus improves polyketide biosynthesis (Fig. 6). The two PKS TEIIs FscTE and TylO were characterized, in this work, with remarkable hydrolytic activities for the nonelongatable acetyl and propionyl thioesters but very low activities for the correct malonyl and methylmalonyl thioesters required for FR-008/candicidin and tylosin biosyntheses. Correspondingly, another PKS TEII, PikAV, was also demonstrated with a distinct hydrolysis preference for propionyl-ACP (kcat/Km, 15.8 ± 1.8 M−1 s−1) over methylmalonyl-ACP (kcat/Km, 3.3 ± 1.1 M−1 s−1) (18).
FIG. 6.
FscTE specifically removes the nonelongatable residues (acetyl or propionyl) bound to ACP in PKS elongation steps.
Interestingly, PikAV seems to have adverse activity to polyketide biosynthesis as it can cleave the malonyl thioester group from ACP with comparable kcat/Km value to acetyl thioester group (18). Thus, the adverse activity of TEII was assumed to be responsible for the reduced product yield after overexpression of PikAV TEII. In contrast to the adverse effect of PikAV overexpression, enhanced expression of FscTE did not suppress FR-008/candicidin formation, supporting the notion that TEIIs are only specifically responsible for the removal of aberrant residues from ACP rather than disturbing the normal process. Additionally, the adverse effect of TEII had not been found in NRPS systems so far, where TEIIs were also reported to improve polypeptide biosynthesis by hydrolytically removing the unprocessed acetyl or aminoacetyl residues from misprimed NRPS proteins (31, 40). In the in vitro simulation of NRPS assembly reaction, SrfAD TEII did not suppress the tripeptide formation, and also, no disturbing effect was observed while the YbtT TEII was incubated, at 10-fold molar excess, with the in vitro reaction of a reconstituted yersiniabactin NRPS/PKS assembly line (24, 31). As a futile reaction, TEII-catalyzed hydrolysis of the elongatable substrates should not be the physiological role of TEIIs.
We also compared the restored product yields in the fscTE mutant by combining fscTE with either the strong constitutive promoter (ermE*p) or the fsc promoters (fscDp and fscMIp). The constitutive ermE*p promoter was found to be more efficient than the native promoters fscDp and fscMIp from FR-008/candicidin gene cluster (Fig. 4B), suggesting that the constitutive expression of fscTE can well maintain the efficiency of FR-008/candicidin biosynthesis.
In accordance with previous studies (13, 18, 31, 40), the two TEIIs characterized were also demonstrated with high Km values (around 33 mM) to acyl thioesters, which implies low affinity of TEIIs for acyl-ACP. However, compared to the carboxylated acyl-ACP units processed readily by the PKS, the aberrant residues bound to unprocessed PKS protein with an increased half-life may serve as better substrates for TEII in vivo (13, 18).
The nonequivalent catalysis of TEIIs from PKSs and NRPSs has been distinctively presented in the text. TylO (13) restored 30% productivity of FR-008/candicidin in the fscTE mutant, whereas two NRPS TEIIs, SrfAD and TycF (40), were null for restoring production. NbmB, the PKS TEII, was also reported to be successful in complementing the tylO mutant for tylosin production (3). In the biochemical assay, SrfAD and BacT, the NRPS TEIIs, were exhibited with hydrolytic activity to acetyl-PCP but with no activity to acetyl-ACP (31). In addition, PikAV, the PKS TEII, has comparable activities toward the methylmalonyl thioester bound to the ACPs derived from either PikAIII or DEBS1 (18). The nonequivalent catalysis of TEIIs from PKSs and NRPSs might attribute to their recognition to ACP or PCP.
TEII seems to be generally required for maximizing the efficiencies of polyketide or oligopeptide biosynthesis, since the intrinsic errors occurred in PKSs or NRPSs (13, 18, 31, 40) and the TEII genes associated with many of their gene clusters (3-6, 16, 20, 21, 30). The absence of TEII genes in some gene clusters (36, 42) may be compensated for by their homologs associated with other clusters elsewhere in chromosome, as many streptomycetes usually contain dozens of distinct biosynthetic gene clusters (1, 25, 26, 36).
Supplementary Material
Acknowledgments
We thank Christopher T. Walsh for providing plasmids pET30a(TycF) and pET30a(srfA-D) and Peter F. Leadlay for providing plasmids pMLH27 and pIB139. We thank David E. Cane for the gift of SNAC thioester samples. We also thank Russell J. Cox for providing the procedure of preparing malonyl-SNAC and Weiguo He for suggestions on methylmalonyl-SNAC synthesis.
This work received financial support from the National Science Foundation of China, the Ministry of Science and Technology (973 and 863 Programs), the Shanghai Municipal Council of Science and Technology, and Shanghai Leading Academic Discipline Project B203.
Footnotes
Published ahead of print on 3 October 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 2.Bibb, M. J., G. R. Janssen, and J. M. Ward. 1985. Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene 38:215-226. [DOI] [PubMed] [Google Scholar]
- 3.Butler, A. R., N. Bate, and E. Cundliffe. 1999. Impact of thioesterase activity on tylosin biosynthesis in Streptomyces fradiae. Chem. Biol. 6:287-292. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S., X. Huang, X. Zhou, L. Bai, J. He, K. J. Jeong, S. Y. Lee, and Z. Deng. 2003. Organizational and mutational analysis of a complete FR-008/candicidin gene cluster encoding a structurally related polyene complex. Chem. Biol. 10:1065-1076. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S., J. B. Roberts, Y. Xue, D. H. Sherman, and K. A. Reynolds. 2001. The Streptomyces venezuelae pikAV gene contains a transcription unit essential for expression of enzymes involved in glycosylation of narbonolide and 10-deoxymethynolide. Gene 263:255-264. [DOI] [PubMed] [Google Scholar]
- 6.Doi-Katayama, Y., Y. J. Yoon, C. Y. Choi, T. W. Yu, H. G. Floss, and C. R. Hutchinson. 2000. Thioesterases and the premature termination of polyketide chain elongation in rifamycin B biosynthesis by Amycolatopsis mediterranei S699. J. Antibiot. (Tokyo) 53:484-495. [DOI] [PubMed] [Google Scholar]
- 7.Donadio, S., M. J. Staver, J. B. McAlpine, S. J. Swanson, and L. Katz. 1991. Modular organization of genes required for complex polyketide biosynthesis. Science 252:675-679. [DOI] [PubMed] [Google Scholar]
- 8.Finking, R., and M. A. Marahiel. 2004. Biosynthesis of nonribosomal peptides. Annu. Rev. Microbiol. 58:453-488. [DOI] [PubMed] [Google Scholar]
- 9.Geoffroy, V. A., J. D. Fetherston, and R. D. Perry. 2000. Yersinia pestis YbtU and YbtT are involved in synthesis of the siderophore yersiniabactin but have different effects on regulation. Infect. Immun. 68:4452-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert, I. H., M. Ginty, J. A. O'Neill, T. A. Simpson, J. Staunton, and C. L. Willis. 1995. Synthesis of β-keto and α,β-unsaturated N-acetylcysteamine thioesters. Bioorg. Med. Chem. 5:1587-1590. [Google Scholar]
- 11.Gokhale, R. S., D. Hunziker, D. E. Cane, and C. Khosla. 1999. Mechanism and specificity of the terminal thioesterase domain from the erythromycin polyketide synthase. Chem. Biol. 6:117-125. [DOI] [PubMed] [Google Scholar]
- 12.Harvey, B. M., H. Hong, M. A. Jones, Z. A. Hughes-Thomas, R. M. Goss, M. L. Heathcote, V. M. Bolanos-Garcia, W. Kroutil, J. Staunton, P. F. Leadlay, and J. B. Spencer. 2006. Evidence that a novel thioesterase is responsible for polyketide chain release during biosynthesis of the polyether ionophore monensin. Chembiochem 7:1435-1442. [DOI] [PubMed] [Google Scholar]
- 13.Heathcote, M. L., J. Staunton, and P. F. Leadlay. 2001. Role of type II thioesterases: evidence for removal of short acyl chains produced by aberrant decarboxylation of chain extender units. Chem. Biol. 8:207-220. [DOI] [PubMed] [Google Scholar]
- 14.Hopwood, D. A. 1997. Genetic contributions to understanding polyketide synthases. Chem. Rev. 97:2465-2498. [DOI] [PubMed] [Google Scholar]
- 15.Hu, Z., K. Bao, X. Zhou, Q. Zhou, D. A. Hopwood, T. Kieser, and Z. Deng. 1994. Repeated polyketide synthase modules involved in the biosynthesis of a heptaene macrolide by Streptomyces sp. FR-008. Mol. Microbiol. 14:163-172. [DOI] [PubMed] [Google Scholar]
- 16.Hu, Z., B. A. Pfeifer, E. Chao, S. Murli, J. Kealey, J. R. Carney, G. Ashley, C. Khosla, and C. R. Hutchinson. 2003. A specific role of the Saccharopolyspora erythraea thioesterase II gene in the function of modular polyketide synthases. Microbiology 149:2213-2225. [DOI] [PubMed] [Google Scholar]
- 17.Janssen, G. R., and M. J. Bibb. 1993. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene 124:133-134. [DOI] [PubMed] [Google Scholar]
- 18.Kim, B. S., T. A. Cropp, B. J. Beck, D. H. Sherman, and K. A. Reynolds. 2002. Biochemical evidence for an editing role of thioesterase II in the biosynthesis of the polyketide pikromycin. J. Biol. Chem. 277:48028-48034. [DOI] [PubMed] [Google Scholar]
- 19.Kohli, R. M., J. W. Trauger, D. Schwarzer, M. A. Marahiel, and C. T. Walsh. 2001. Generality of peptide cyclization catalyzed by isolated thioesterase domains of nonribosomal peptide synthetases. Biochemistry 40:7099-7108. [DOI] [PubMed] [Google Scholar]
- 20.Kotowska, M., K. Pawlik, A. R. Butler, E. Cundliffe, E. Takano, and K. Kuczek. 2002. Type II thioesterase from Streptomyces coelicolor A3(2). Microbiology 148:1777-1783. [DOI] [PubMed] [Google Scholar]
- 21.Linne, U., D. Schwarzer, G. N. Schroeder, and M. A. Marahiel. 2004. Mutational analysis of a type II thioesterase associated with nonribosomal peptide synthesis. Eur. J. Biochem. 271:1536-1545. [DOI] [PubMed] [Google Scholar]
- 22.Liu, T., D. You, C. Valenzano, Y. Sun, J. Li, Q. Yu, X. Zhou, D. E. Cane, and Z. Deng. 2006. Identification of NanE as the thioesterase for polyether chain release in nanchangmycin biosynthesis. Chem. Biol. 13:945-955. [DOI] [PubMed] [Google Scholar]
- 23.Lu, H., S. C. Tsai, C. Khosla, and D. E. Cane. 2002. Expression, site-directed mutagenesis, and steady state kinetic analysis of the terminal thioesterase domain of the methymycin/picromycin polyketide synthase. Biochemistry 41:12590-12597. [DOI] [PubMed] [Google Scholar]
- 24.Miller, D. A., L. Luo, N. Hillson, T. A. Keating, and C. T. Walsh. 2002. Yersiniabactin synthetase: a four-protein assembly line producing the nonribosomal peptide/polyketide hybrid siderophore of Yersinia pestis. Chem. Biol. 9:333-344. [DOI] [PubMed] [Google Scholar]
- 25.Oliynyk, M., M. Samborskyy, J. B. Lester, T. Mironenko, N. Scott, S. Dickens, S. F. Haydock, and P. F. Leadlay. 2007. Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol. 25:447-453. [DOI] [PubMed] [Google Scholar]
- 26.Omura, S., H. Ikeda, J. Ishikawa, A. Hanamoto, C. Takahashi, M. Shinose, Y. Takahashi, H. Horikawa, H. Nakazawa, T. Osonoe, H. Kikuchi, T. Shiba, Y. Sakaki, and M. Hattori. 2001. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA 98:12215-12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeifer, B., Z. Hu, P. Licari, and C. Khosla. 2002. Process and metabolic strategies for improved production of Escherichia coli-derived 6-deoxyerythronolide B. Appl. Environ. Microbiol. 68:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quadri, L. E., P. H. Weinreb, M. Lei, M. M. Nakano, P. Zuber, and C. T. Walsh. 1998. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 37:1585-1595. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Schneider, A., and M. A. Marahiel. 1998. Genetic evidence for a role of thioesterase domains, integrated in or associated with peptide synthetases, in non-ribosomal peptide biosynthesis in Bacillus subtilis. Arch. Microbiol. 169:404-410. [DOI] [PubMed] [Google Scholar]
- 31.Schwarzer, D., H. D. Mootz, U. Linne, and M. A. Marahiel. 2002. Regeneration of misprimed nonribosomal peptide synthetases by type II thioesterases. Proc. Natl. Acad. Sci. USA 99:14083-14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarzer, D., H. D. Mootz, and M. A. Marahiel. 2001. Exploring the impact of different thioesterase domains for the design of hybrid peptide synthetases. Chem. Biol. 8:997-1010. [DOI] [PubMed] [Google Scholar]
- 33.Schwecke, T., J. F. Aparicio, I. Molnar, A. Konig, L. E. Khaw, S. F. Haydock, M. Oliynyk, P. Caffrey, J. Cortes, J. B. Lester, et al. 1995. The biosynthetic gene cluster for the polyketide immunosuppressant rapamycin. Proc. Natl. Acad. Sci. USA 92:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma, K. K., and C. N. Boddy. 2007. The thioesterase domain from the pimaricin and erythromycin biosynthetic pathways can catalyze hydrolysis of simple thioester substrates. Bioorg. Med. Chem. Lett. 17:3034-3037. [DOI] [PubMed] [Google Scholar]
- 35.Short, J. M., J. M. Fernandez, J. A. Sorge, and W. D. Huse. 1988. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 16:7583-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, Y., X. Zhou, J. Liu, K. Bao, G. Zhang, G. Tu, T. Kieser, and Z. Deng. 2002. ‘Streptomyces nanchangensis,’ a producer of the insecticidal polyether antibiotic nanchangmycin and the antiparasitic macrolide meilingmycin, contains multiple polyketide gene clusters. Microbiology 148:361-371. [DOI] [PubMed] [Google Scholar]
- 37.Tang, L., H. Fu, M. C. Betlach, and R. McDaniel. 1999. Elucidating the mechanism of chain termination switching in the picromycin/methymycin polyketide synthase. Chem. Biol. 6:553-558. [DOI] [PubMed] [Google Scholar]
- 38.Tsai, S. C., L. J. Miercke, J. Krucinski, R. Gokhale, J. C. Chen, P. G. Foster, D. E. Cane, C. Khosla, and R. M. Stroud. 2001. Crystal structure of the macrocycle-forming thioesterase domain of the erythromycin polyketide synthase: versatility from a unique substrate channel. Proc. Natl. Acad. Sci. USA 98:14808-14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson, C. J., Z. A. Hughes-Thomas, C. J. Martin, I. Bohm, T. Mironenko, M. Deacon, M. Wheatcroft, G. Wirtz, J. Staunton, and P. F. Leadlay. 2002. Increasing the efficiency of heterologous promoters in actinomycetes. J. Mol. Microbiol. Biotechnol. 4:417-426. [PubMed] [Google Scholar]
- 40.Yeh, E., R. M. Kohli, S. D. Bruner, and C. T. Walsh. 2004. Type II thioesterase restores activity of a NRPS module stalled with an aminoacyl-S-enzyme that cannot be elongated. Chembiochem 5:1290-1293. [DOI] [PubMed] [Google Scholar]
- 41.Yu, F. M., B. Qiao, F. Zhu, J. C. Wu, and Y. J. Yuan. 2006. Functional analysis of type II thioesterase of Streptomyces lydicus AS 4.2501. Appl. Biochem. Biotechnol. 135:145-158. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, C., J. Ju, S. D. Christenson, W. C. Smith, D. Song, X. Zhou, B. Shen, and Z. Deng. 2006. Utilization of the methoxymalonyl-acyl carrier protein biosynthesis locus for cloning the oxazolomycin biosynthetic gene cluster from Streptomyces albus JA3453. J. Bacteriol. 188:4142-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou, Y., L. J. Zhu, J. Chen, S. Bai, L. Zhou, X. Wu, and H. Z. Deng. 2008. Jun. Incomplete beta-ketone processing as a mechanism for polyene structural variation in the FR-008/candicidin complex. Chem. Biol. 15:629-638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.