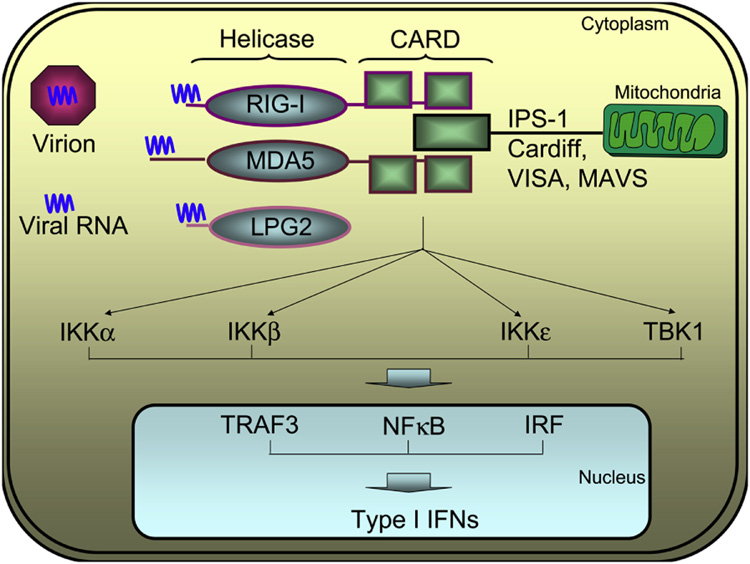
Figure 3. Intracytoplasmic viral recognition and antiviral pathways.
The RNA derived from virions is recognized at the helicase domain of the RIG-I, MDA5 or LPG2 helicases. Upon ligand interaction, the tandem caspase activation and recruitment (CARD) domains of RIG-I and MDA5 engage the CARD domain of mitochondria-bound IPS-1 and trigger activation of a series of kinases including IKKα, IKKβ, IKKε and TBK1. These signaling events activate TRAF3, NFκB and IRF, leading to their translocation to the nucleus and initiation of the type 1 IFN production. LPG2 is CARD-less and does not trigger the signaling events, however it modulates the activity of RIG-I by blocking RIG-I self-association, owed to disrupting homotypic CARD/helicase domain and/or C terminus interactions, by disrupting assembly of the RIG-I-containing signaling complex on IPS-1 and by sequestering the viral dsRNA.