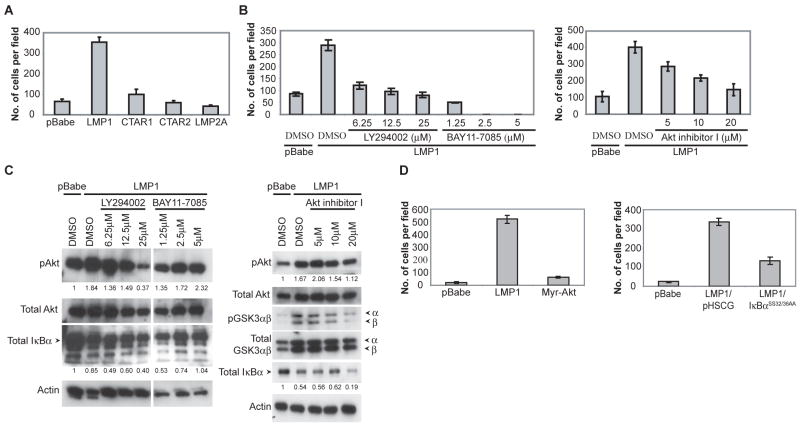
Figure 5.
LMP1 induced migration requires both CTAR1 and 2 domains and is dependent on PI3K and NFκB signaling. (A) C666-1 cells stably expressing LMP1, CTAR1, CTAR2, LMP2A or the pBabe vector control was assessed for metastasis in the transwell migration assay. (B) The signals required by LMP1 for enhanced migration was assessed by using inhibitors of PI3K (LY294002), Akt (Akt inhibitor I) and NFκB (BAY11-7085) signaling at the indicated concentrations, compared to the DMSO control. (C) The ability of the PI3K inhibitor (LY294002), NFκB inhibitor (BAY11-7085) and Akt inhibitor (Akt inhibitor I) to block PI3K/Akt and IκBα-dependent NFκB signaling was assessed by immunoblot analysis after an overnight treatment, and blotted for activated phosphorylated Akt (pAkt), inactivated phosphorylated GSK3 (pGSK3αβ) and total IκBα levels. Actin was used as a loading control. Densitometry with Image J software was performed to normalize pAkt and IκBα levels to the corresponding actin levels. Fold change of the normalized pAkt and IκBα levels relative to the pBabe control is indicated below the immunoblots. White line indicates that intervening lanes have been spliced out. (D) LMP1-induced migration is dependent on IκBα-dependent NFκB signaling and activation of Akt is not sufficient to enhance migration. Expression of the constitutively active myristylated Akt (myr-Akt) was used to assess whether activation of Akt signaling is sufficient to enhance migration. The requirement of canonical NFκB signaling for LMP1-induced migration was assessed using the IκBα super-repressor (IκBαSS32/36AA), compared to the pHSCG vector control.