FIG. 1.
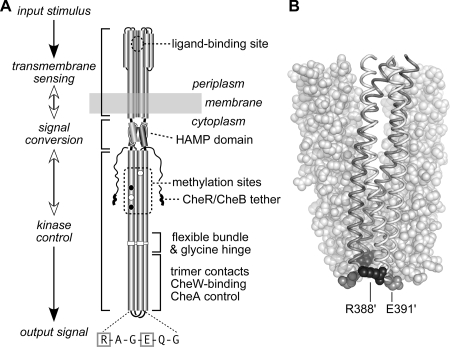
(A) Functional architecture of Tsr and other MCP molecules. Native Tsr is a homodimer; each subunit is 551 residues in length. Most of the secondary-structure elements are alpha helices (shown as thickened cylinders). Structural elements of the transmembrane-sensing, HAMP, and kinase control domains are shown in their native arrangement. The kinase control domain is organized as a four-helix bundle of coiled coils. The Tsr residues in the hairpin loop at the cytoplasmic tip of the coiled-coil bundle are shown: the boxed residues (R388 and E391) are the subjects of the present study. Several of the adaptation (methylation) sites are initially synthesized as glutamate residues (white circles); others are synthesized as glutamine residues (black circles) and subsequently converted to glutamates by an irreversible CheB-mediated deamidation. The C terminus of each subunit ends in a pentapeptide (NWETF) to which the adaptation enzymes, CheR and CheB, can bind. An ∼30-residue segment, thought to serve as a flexible linker, joins this tethering site to the kinase control domain. (B) Structure of the Tsr trimer of dimers. This view corresponds to the flexible-bundle and trimer contact portion of the dimer schematic in panel A, with the membrane-distal tip at the bottom. Two dimers are shown space filled (white); the third is shown as a backbone trace (gray and white subunits) with key residues in space fill mode: R388 (black) and E391 (gray). Note that R388 in the gray subunit lies (mostly hidden) at the trimer interface, whereas its counterpart in the white subunit lies at the trimer perimeter. The convention used throughout this report is to designate residues in the nonaxial subunits of the trimer with primes, e.g., R388′ and E391′.