Abstract
The central role of plasmacytoid dendritic cells (pDC) in activating host immune responses stems from their high capacity to express alpha interferon (IFN-α) after stimulation of Toll-like receptors 7 and 9 (TLR7 and -9). This involves the adapter MyD88 and the kinases interleukin-1 receptor-associated kinase 1 (IRAK1), IRAK4, and IκB kinase α (IKKα), which activates IFN regulatory factor 7 (IRF7) and is independent of the canonical kinases TBK1 and IKKɛ. We have recently shown that the immunosuppressive measles virus (MV) abolishes TLR7/9/MyD88-dependent IFN induction in human pDC (Schlender et al., J. Virol. 79:5507-5515, 2005), but the molecular mechanisms remained elusive. Here, we have reconstituted the pathway in cell lines and identified IKKα and IRF7 as specific targets of the MV V protein (MV-V). Binding of MV-V to IKKα resulted in phosphorylation of V on the expense of IRF7 phosphorylation by IKKα in vitro and in living cells. This corroborates the role of IKKα as the kinase phosphorylating IRF7. MV-V in addition bound to IRF7 and to phosphomimetic IRF7 and inhibited IRF7 transcriptional activity. Binding to both IKKα and IRF7 required the 68-amino-acid unique C-terminal domain of V. Inhibition of TLR/MyD88-dependent IFN induction by MV-V is unique among paramyxovirus V proteins and should contribute to the unique immunosuppressive phenotype of measles. The mechanisms employed by MV-V inspire strategies to interfere with immunopathological TLR/MyD88 signaling.
Measles virus (MV) is an important human pathogen that induces a generalized transient immune suppression which accounts for most of the mortality associated with measles as well as a specific immune response that provides life-long protection (41). This paradoxical effect is most likely due to early interactions of MV with cells of the immune system, including conventional and plasmacytoid dendritic cells (cDC and pDC, respectively). Manifold functions of DC are compromised by MV infection, which is proposed to contribute to immune suppression (19). A particularly remarkable feature of MV is the ability to shut down alpha interferon (IFN-α) production in response to Toll-like receptor 7 (TLR7) and TLR9 ligands in infected human pDC in vitro, as we could show previously (48).
Human pDC are responsible for the bulk of early IFN production in response to virus infection and for stimulation of important adaptive immune response mechanisms (for reviews, see references 8 and 18). The capacity of pDC for instant expression of huge amounts of IFN-α stems from a special signaling cascade activating IFN regulatory factor 7 (IRF7) upon TLR7/9 stimulation (25, 33). This relies on the presence of latent IRF7 (26) and high-level expression of the endosomal TLR7 and -9 (28). Agonists of TLR7 and -9 include single-stranded RNA (ssRNA) and DNA, respectively, as well as nucleic acid derivatives which are being used as immune modulators and adjuvants (22, 23). After ligation of TLR7/9, IRF7 is recruited and phosphorylated in a complex including the Toll-interleukin-1 (IL-1) receptor (TIR) adapter MyD88, the E3-ubiquitin ligases tumor necrosis factor receptor-associated factor 3 (TRAF3) and TRAF6, and the kinases IL-1 receptor-associated kinase 1 (IRAK1) and IRAK4 (24, 25, 33, 34, 59, 60). Although IRAK1 was originally implicated in phosphorylation of IRF7, a recent report suggested this role for IκB kinase α (IKKα) (29). Phosphorylated IRF7 dimerizes and is translocated to the nucleus, where it binds to the promoter of IFN-α genes and upregulates their expression. Importantly, not only exogenous endocytosed nucleic acids may stimulate TLR7/9 IFN signaling, but also intracellular cytoplasmic nucleic acids that have been engulfed by the process of autophagy and delivered to endosomes (37). In turn, TLR signaling stimulates autophagy, thereby improving recognition of cytoplasmic viral RNAs (14).
Apart from virus recognition by pDC and induction of antiviral immunity, a critical role of TLR7/9 signaling in amplifying autoimmune responses and sustaining autoimmune conditions has been established. Recent studies have revealed inappropriate activation of IFN by TLR7/9 in systemic lupus erythematosus and several other autoimmune diseases. Intriguingly, in this respect, it was shown that also cDC and macrophages can produce high levels of IFN when the spatiotemporal regulation of MyD88/IRF7 signaling is affected (24, 57), specifically, when the TLR ligands are retained in or redirected to endosomes by autoimmune complexes containing RNA and DNA (for recent reviews, see references 35 and 36).
In order to further address TLR7-MyD88-dependent IFN-α induction and to reveal the counteracting mechanism(s) evolved by MV, we have restored the pathway in cell lines by expression of individual components from transfected plasmids. Biochemical and reporter gene assays strongly support the direct involvement of IKKα in IRF7 phosphorylation, which is corroborated by the identification of IKKα as a specific molecular target of the MV protein V (MV-V).
MV-V is expressed from the MV phosphoprotein (MV-P) gene by mRNA editing (9) and is composed of an N-terminal sequence identical to that of the MV-P and a unique 68-amino-acid (aa) Cys-rich and zinc-binding C-terminal domain (39, 45). The MV-V-specific C terminus was identified here as an autonomous targeting module directing V to IKKα and IRF7, while sequences of the common N-terminal domain were necessary for potent inhibition of IRF7-mediated IFN-α promoter activation. Among the V proteins of paramyxoviruses, which share organization and several immune escape functions, MV-V is unique in targeting MyD88-dependent IKKα/IRF7 activation. It is strongly suggested that this peculiar function of MV-V contributes to the peculiar immunopathology of measles.
MATERIALS AND METHODS
Cell lines.
HEK-293, 293T, and Huh7.5 cells (kindly provided by C. Rice) were propagated in Dulbecco's minimal essential medium with 10% fetal bovine serum, 1× l-glutamine, and penicillin-streptomycin (Invitrogen). BSR-T7/5 cells were propagated in Glasgow minimal essential medium with 10% newborn calf serum, 1× nonessential amino acids, 1× tryptose-phosphate, and penicillin-streptomycin.
cDNA constructs.
Open reading frames (ORFs) encoding MV-P and MV-V (Schwarz vaccine strain) were cloned in pCR3 (Invitrogen). Expression of the C protein was abolished in pCR3-PΔC and pCR3-VΔC by exchange of three nucleotides by site-directed mutagenesis using primers 1 and 2 (Table 1) and leading to disruption of the start codon and introduction of two stop codons.
TABLE 1.
Primers used for cloning of cDNAs
| Primer no. | Primer name | Sequencea |
|---|---|---|
| 1 | MV-PVΔC-fwd | AGAGCAGGCACGCCACGTGAAAAACGGACTAGAATGCATCC |
| 2 | MV-PVΔC-rev | TGGCGTGCCTGCTCTTCTGCCATGG |
| 3 | MV-PVN-fwd | ATAGAATTCGCCACCATGGCAGAAGAGCAGGCA |
| 4 | MV-PVN-rev | ATACTCGAGTTACCCCTTTTTAATGGG |
| 5 | MV-PC-fwd | ATAGAATTCGCCACCATGACAGACGCGAGATTAGCC |
| 6 | MV-PC-rev | ATACTCGAGCTACTTCATTATTATCTT |
| 7 | MV-VC-fwd | ATAGAATTCGCCACCATGCACAGACGCGAGATTAGC |
| 8 | MV-VC-rev | ATACTCGAGTTATTCTGGGATCTCGGG |
| 9 | MV-V-full_length-fwd | ATAGCTAGCATGGCAGAAGAGCAGGCA |
| 10 | MV-V-full_length-rev | TGCGGCCGCTTATTCTGGGATCTC |
| 11 | IRF7-fwd | ATAGCTAGCATGGCTGAAGTGAGGGGG |
| 12 | IRF7-rev | ATATGCGGCCGCTCAAGGCCACTGACC |
Exchanged nucleotides are underlined.
Immunoglobulin (Ig)-tagged versions of MV-P, MV-V, and MV-C were generated by cloning EcoRI/NotI restriction fragments of pCR3-PΔC, pCR3-VΔC, and pCR3-MV-C into pCR3-Ig (a modified pCR3 vector expressing an Ig tag upstream of EcoRI). Ig-tagged protein domains MV-PVN (aa 1 to 231), MV-PC (aa 232 to 507), and MV-VC (aa 232 to 299) were generated by cloning PCR products from pCR3-PΔC and pCR3-VΔC into pCR3-Ig (EcoRI/XhoI) using primers 3 to 8.
To generate vectors for bacterial expression, ORFs of MV-P, MV-V, and IRF7 were cloned in pET28a (Novagen), using the NheI/NotI restriction sites and primers 9 to 12.
Bacterial expression and Ni-NTA purification.
His-MV-P, His-MV-V, and His-IRF7 were expressed in Escherichia coli BL21 Rosetta cells (Novagen) and purified by Ni-nitrilotriacetic acid (NTA) affinity chromatography as described elsewhere (13).
Transfection.
For reporter gene assays, 1 × 105 cells (Huh7.5, 293, or 293T) were seeded in 24-well microtiter plates. After 16 h, 100 ng of the firefly luciferase (FL) reporter plasmid p55C1B-Luc (kindly provided by T. Fujita), IFNα4-Luc, or IFNα6-Luc (kindly provided by S. Akira) was transfected using Lipofectamine 2000 transfection reagent (Invitrogen). As an internal control, 10 ng of pCMV-RL was cotransfected in all experiments. Various amounts of pCR3-PΔC, pCR3-VΔC, myc-MyD88 (K. Ruckdeschel), Fl-TRAF6 (A. Kieser), Fl-IKKα (K. Ruckdeschel), Fl-IRF7 (murine from S. Akira and human from J. Hiscott), Fl-IRAK1, Fl-IRAK1c, Fl-IRAK4 (all from RZPD), and Fl-TBK1 were cotransfected as indicated. Amounts of transfected DNA were adjusted by adding pCR3 or pCR3-Ig vector.
For coimmunoprecipitation experiments, HEK-293T cells were transfected in 21-cm2 dishes (2.6 × 106 cells/dish) with 2 μg of Fl-IKKα, Fl-IKKβ (K. Ruckdeschel), Fl-IKKɛ (J. Hiscott), Fl-TBK1, Fl-IRF7, Fl-IRF7-2D, Fl-IRF3, or Fl-IRF3-5D (J. Hiscott), as well as 2 μg of Ig-tagged P/V/C constructs or domains or with pCR3-Ig empty vector as indicated. Pull-down experiments were performed 24 h posttransfection as previously described (5) using protein A-conjugated Sepharose beads (GE Healthcare) to pull down Ig-tagged proteins or anti-FLAG M2 affinity gel (Sigma) to pull down Flag-tagged proteins.
Luciferase assay.
Cell lysates were prepared 24 h posttransfection and subjected to reporter gene assay using the Promega dual-luciferase reporter system. Luciferase activity was measured with a Luminometer (Berthold Centro LB 960 or Berthold Lumat LB 9501) according to the supplier's instructions. Graphs show mean values of at least three independent experiments plus standard deviations.
Western blots and antibodies.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed as previously described (5). Anti-MV-P (C terminus; #37069) was kindly provided by D. Gerlier (10), anti-MV-P (N terminus; P3) was kindly provided by S. Schneider-Schaulies, and anti-MV-V (C terminus) and anti-MV-C were kindly provided by R. Cattaneo. Anti-Flag-M2 and anti-His were purchased from Sigma and Cell Signaling, respectively.
Blots shown are representative of at least three independently performed experiments.
In vitro kinase assays.
Kinase-active His-IKKα (100 ng/reaction [Invitrogen]) was mixed with purified His-IRF7 (500 ng/reaction) or glutathione S-transferase (GST)-IκB-α (100 ng/reaction [Santa Cruz]) and increasing amounts of His-MV-V or His-MV-P (0 to 1,600 pmol/reaction as indicated) in reaction buffer (50 mM HEPES, 0,01% Triton X-100, 10 mM MgCl2, 1 mM EGTA, 0.5 mM Na3VO4, 5 mM β-glycerophosphate, 2 mM dithiothreitol [DTT], pH 7.5). To start the reaction, 200 μM ATP with tracing amounts of [γ-32P]ATP (Amersham) were added to the reaction mix. The reaction was stopped by adding denaturing cell lysis buffer. Probes were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Radioactively labeled proteins were detected by autoradiography using a Storm phosphorimager (GE Healthcare) and quantified by ImageQuant software (Molecular Dynamics).
RESULTS
Reconstitution of MyD88-dependent IFN-α activation in cell lines.
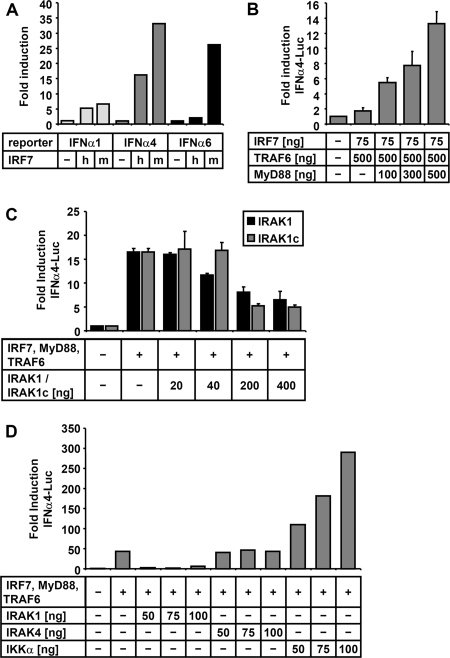
As the TLR7/9-mediated IFN-α-inducing signaling pathway is operating efficiently only in primary pDC, which are less amenable to biochemical approaches, we sought to facilitate the analysis of the pathway by reconstitution in cell lines including BSR-T7/5, HEK-293, HEK-293T, and Huh7.5. First, activation of different IFN-α promoters (α1, α4, and α6) after expression of human IRF7 (hIRF7) and mouse IRF7 (mIRF7) from transfected plasmids was determined (Fig. 1A). The activity of IFNα4- and IFNα6 promoter-controlled firefly luciferase (FL) was conspicuously greater than that of the IFNα1 promoter. In further experiments, the IFNα4 promoter was used in combination with mIRF7, which activated all three promoters better than hIRF7.
FIG. 1.
Reconstitution of MyD88-dependent TLR7/9 signaling in cell lines. (A) 293T cells were transfected with the indicated plasmids, and activation of IFN-α promoters was determined by dual-luciferase assay. Overexpression of mIRF7 (m) and hIRF7 (h) (450 ng of plasmid) leads to efficient induction of the IFNα4 and IFNα6 promoters and moderate induction of IFNα1. (B) TRAF6 and MyD88 expression increases the activity of mIRF7 in a dose-dependent manner. (C) Expression of IRAK1 or IRAK1c in addition to IRF7, MyD88, and TRAF6 leads to a dose-dependent inhibitory effect on IFNα4 promoter-driven FL activity. (D) Effect of indicated amounts of plasmids encoding for IRAK1, IRAK4, and IKKα. A stimulatory and dose-dependent positive effect is only observed for IKKα. Data are derived from three independent experiments and are represented as means + standard deviations.
To reveal the impact of the TIR adapter protein MyD88, the ubiquitin ligase TRAF6, and the kinases involved in the TLR7/9 pathway, the mIRF7-expressing plasmid was used at low concentrations, to yield an approximately one- to fivefold induction of FL after expression of IRF7 alone. Cotransfection of increasing amounts of the ubiquitous TRAF6 had only weak stimulating effects (Fig. 1B and data not shown); however, additional expression of MyD88 greatly increased the induction of IFNα4-Luc in a dose-dependent manner.
Both IRAK1 and IRAK4 have been described as kinases essential for TLR7/9-mediated IFN induction. Unexpectedly, however, additional expression of IRAK1 showed a substantial and dose-dependent inhibitory effect (Fig. 1C). A splicing variant of IRAK1, IRAK1c, which has been reported to have an inhibitory effect on TLR7/9 signaling (47), showed almost identical inhibition. In contrast to IRAK1, additional expression of IRAK4 was not inhibitory, but a clear stimulatory effect was not observed (Fig. 1D).
In striking contrast to IRAKs, overexpression of IKKα strongly enhanced the basal induction obtained by coexpression of IRF7, MyD88, and TRAF6, indicating that IKKα is directly involved in IRF7 phosphorylation. A transfection cocktail containing plasmids encoding IRF7, MyD88, TRAF6, and IKKα in a ratio of 1:5:5:5 showed specific induction of IFNα4 in different transfectable cell lines, including Huh7.5 and BSR-T7/5 cells which have defects in RIG-I- mediated TBK1-dependent IFN induction (see below). Yet, the amount of plasmid mix necessary for high and reliable induction varied in different cell lines from 100 to 600 ng in total (data not shown).
Expression of MV-P gene products.
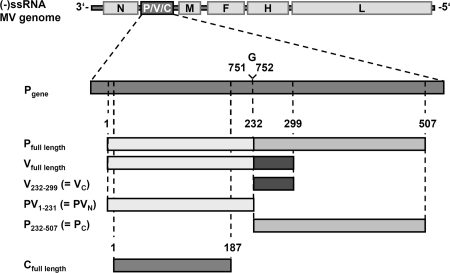
In order to determine whether MV-P gene products are involved in the observed inhibition of TLR7/9 signaling in MV-infected pDC (48), we constructed cDNAs for individual expression of MV-P and -V, as well as the C protein (Fig. 2), which is produced by ribosomal leaky scanning to a second ORF downstream of the P/V start codon (3). While C has been described as an infectivity factor (15), its impact on the IFN response remains controversial (42, 53, 56). In case of all P- or V-expressing plasmids, the start codon of the C ORF was changed by site-directed silent mutagenesis to prevent expression of C by ribosomal scanning. P- and C-expressing plasmids were directly derived from reverse transcription-PCR of P mRNA from MV Schwarz-infected Vero cells, and V cDNA was generated by insertion of a single G residue at the P mRNA editing site. In addition, expression vectors for distinct P or V domains were constructed: MV-PVN, including the N-terminal domain common to P and V (aa 1 to 231); MV-PC, comprising the P-specific C-terminal domain (aa 232 to 507); and MV-VC, comprising the V-specific C-terminal domain (aa 232 to 299). As observed in Western blot experiments with P-, V-, and C-specific antisera, proteins of the expected specificity and size were expressed from transfected pCR3 vector plasmids in 293T cells (not shown). In addition to the authentic proteins, constructs with N-terminal Ig, Flag, or His6 tags were generated.
FIG. 2.
MV-P gene products and cDNA constructs. The P gene of MV is located at the second position of the 16-kb MV negative-sense ssRNA [(−)ssRNA] genome. The P mRNA consists of 1,685 bases; insertion of an additional guanosine between bases 751 and 752 by RNA editing gives rise to the V mRNA. V and P proteins share a common N-terminal protein sequence (PVN, aa 1 to 231) but have unique C termini (PC, aa 232 to 507; VC, aa 232 to 299). cDNAs encoding authentic or tagged P, V, PVN, PC, and VC were constructed by cloning into different vectors for eukaryotic or prokaryotic expression. Expression of the C protein, which is encoded in an alternative ORF, was abolished in the P/V cDNA constructs by site-directed mutagenesis (for details see Materials and Methods) or was expressed from a separate cDNA.
MV-V protein inhibits IKKα-dependent, but not TBK1-dependent, IFN-α induction.
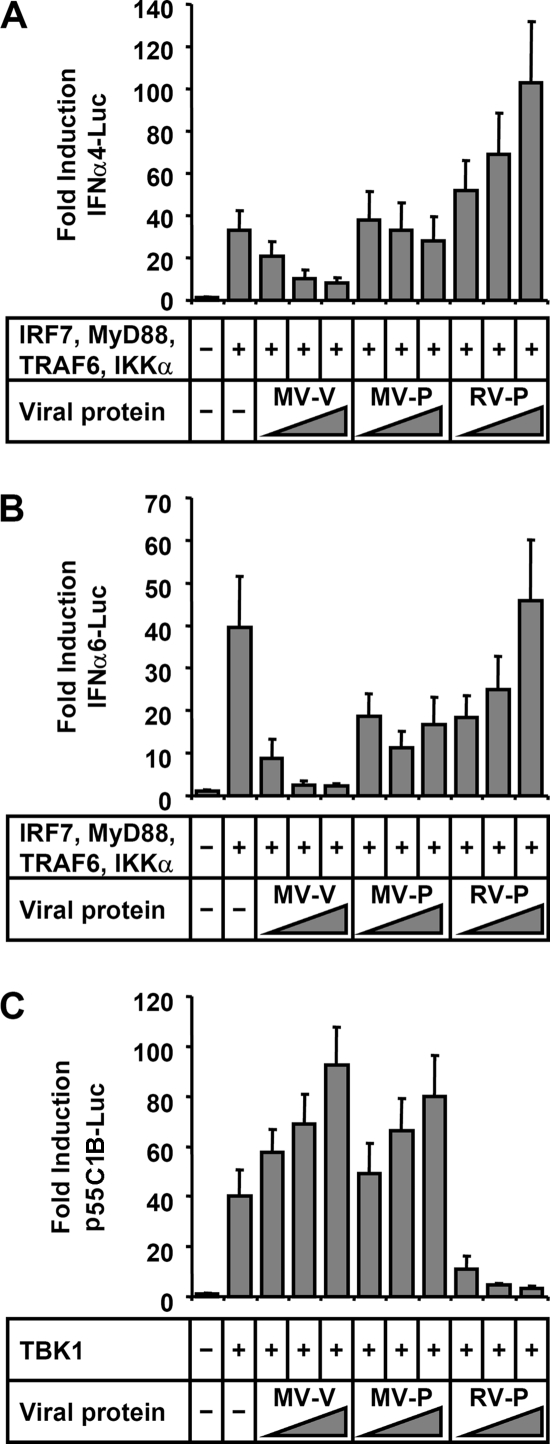
After having established a suitable tool for the analysis of IFN-α induction by the MyD88/IKKα-dependent signaling cascade, we examined the ability of MV proteins to interfere. Experiments were carried out with Huh7.5 cells, as this cell line has a defect in RIG-I signaling (54), thereby minimizing TBK1/IKKɛ-mediated IFN induction. MV-V, MV-P, and the P protein of rabies virus (RV-P), which counteracts TBK1-mediated activation of IRF3 and IRF7, were coexpressed with the stimulating protein mixture described above. In contrast to MV-P and RV-P proteins, MV-V had a substantial and dose-dependent inhibitory effect on the induction of both IFNα4 and IFNα6 promoters (Fig. 3A and B). In parallel experiments, the ability of the viral proteins to counteract TBK1-mediated induction of IFN promoters was addressed. To this end, TBK1 was overexpressed to induce p55C1B (IRF3)-driven FL (Fig. 3C). While RV-P efficiently blocked expression of FL activity, neither of the MV proteins had inhibitory effects. Thus, MV-V protein specifically targets activation of IRF by the TLR7/9- and MyD88-dependent signal cascade, which involves IKKα, but is ineffective in preventing the classical IFN-inducing pathway involving TBK1 and the related IKKɛ.
FIG. 3.
MV-V protein inhibits MyD88-dependent induction of IFN-α promoters. (A and B) Induction of IFNa4 (A) and IFNa6 (B) promoters by IRF7 (10 ng) and MyD88, TRAF6, and IKKα (50 ng each) is inhibited by cotransfection of MV-V (250, 500, and 750 ng) in a dose-dependent manner, in contrast to MV-P and RV-P. (C) Induction of the p55C1B part of the IFN-β promoter by overexpression of TBK1 (150 ng of plasmid) is antagonized by expression of RV-P, but not by MV-V or MV-P. Data are derived from three independent experiments and are represented as means + standard deviations.
MV-V binds to IKKα and IRF7.
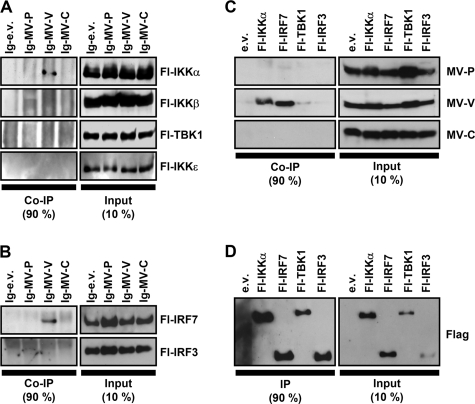
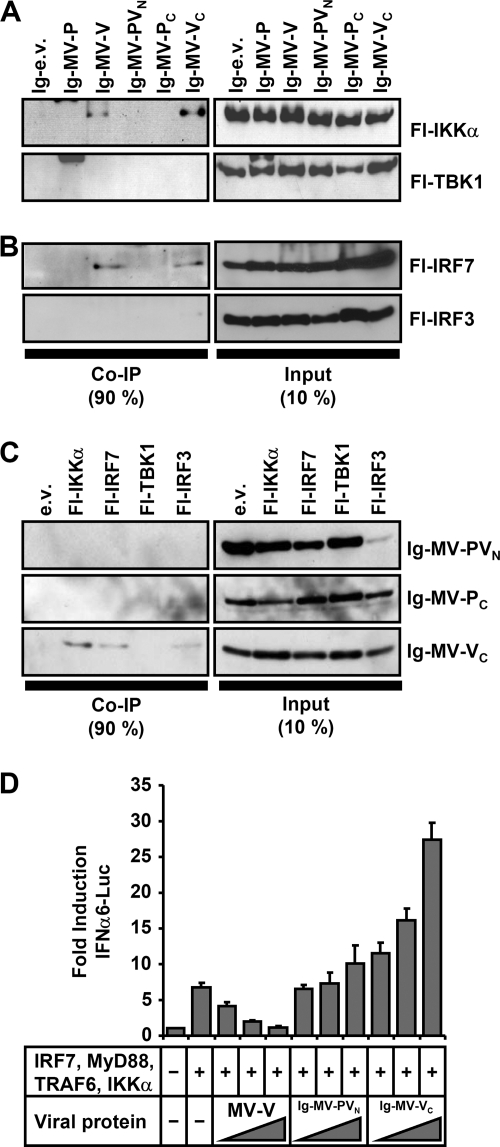
In order to identify the molecular targets of MV-V protein in the TLR7/9-dependent IFN induction pathway, coprecipitation experiments were performed. In a first trial, interaction of MV proteins with the pathway-specific kinase IKKα and its homologues was addressed. Extracts from 293T cells coexpressing Ig-tagged MV proteins and Flag-tagged versions of IKKα, IKKβ, TBK1, and IKKɛ were purified by protein A-conjugated Sepharose beads. Indeed, IKKα specifically copurified with Ig-MV-V, but not with -MV-P, or -MV-C (Fig. 4A). In a similar experiment, the interaction of MV proteins with IRF3 and IRF7 was assessed. Fl-IRF7 was copurified with Ig-MV-V, but not with P or C proteins (Fig. 4B), while Fl-IRF3 did not reveal interactions with either of the viral proteins.
FIG. 4.
Interaction of MV-V with IKKα and IRF7. (A and B) pIg-MV-P, -MV-V, and -MV-C or empty vector (pIg-e.v.) was cotransfected with pFl-IKKα, -IKKβ, -TBK1, -IKKɛ, -IRF7, or -IRF3 in 293T cells as indicated. Cells were lysed 24 h posttransfection under native conditions, and Ig-tagged proteins were pulled down with protein A-conjugated Sepharose beads and subjected to Western blot analysis. MV-V coprecipitated (co-IP) IKKα, but not IKKβ, TBK1, or IKKɛ (A), and in addition, IRF7, but not IRF3 (B). Precipitation of any of the kinases or transcription factors by MV-P or MV-C was not detected. (C and D) Native cell lysates of transfected 293T cells were subjected to anti-Flag affinity gel purification and analyzed by Western blotting. Flag-tagged IKKα, IRF7, TBK1, and IRF3 were efficiently pulled down (D). Only MV-V was coprecipitated by IKKα and IRF7, but not with TBK1 or IRF3 (C).
To confirm the apparently highly specific interactions, complementary pull-down experiments with Fl-IKKα, -IRF7, -TBK1, and -IRF3 were performed. The proteins were purified from cell extracts with anti-Flag M2 affinity gel (Fig. 4D) and analyzed for coprecipitation of authentic, untagged MV-V, -P, and -C proteins. As revealed by Western blot experiments with antibodies specific for MV proteins, both Fl-IKKα and Fl-IRF7 efficiently pulled down MV-V protein (Fig. 4C). For TBK1, a faint signal similar to the empty vector control was observed. Apart from IKKα and IRF7, no interactions of MV-V, -P, or -C protein with other components of the signaling complex such as MyD88 or TRAF6 were indicated by comparable precipitation experiments (data not shown). In summary, the strong physical interaction of MV-V with IKKα and IRF7, but not TBK1 and IRF3, was in line with the observed specificity of blocking TLR9/MyD88-mediated IFN induction.
The C-terminal domain of V is sufficient for interaction with IKKα and IRF7.
As MV-V and -P proteins share an identical N-terminal domain (PVN), the unique MV-V C-terminal domain (VC) was assumed to be key for binding to IKKα and IRF7. The roles of the distinct protein domains were therefore analyzed in protein A-Sepharose pull-down experiments, using Ig-tagged MV-PVN, -PC, and -VC constructs along with the full-length proteins. In fact, Ig-MV-VC was found sufficient for interaction with IKKα. In contrast to Ig-MV-PVN or Ig-MV-PC, Ig-MV-VC very efficiently coprecipitated IKKα, while an interaction with TBK1 was not apparent (Fig. 5A). Similarly, Ig-MV-VC pulled down Fl-IRF7 effectively (Fig. 5B). In reciprocal experiments using the anti-Flag M2 affinity pull-down system, Fl-IKKα and Fl-IRF7 coprecipitated only Ig-MV-VC but not -PVN or -PC (Fig. 5C). In these experiments, a residual interaction of Fl-IRF3 with VC but also PC could not be excluded (Fig. 5C). In summary, the 68-aa C terminus of MV-V is sufficient to autonomously and strongly interact with both IKKα and IRF7.
FIG. 5.
VC mediates interaction with IKKα and IRF7. (A and B) The indicated plasmids or empty vector (e.v.) was cotransfected with pFl-IKKα, -TBK1, -IRF7, or -IRF3 into 293T cells. Ig-tagged proteins were pulled down under native conditions and analyzed by Western blotting for interaction partners. Full-length Ig-MV-V as well as Ig-VC was identified to efficiently bind IKKα, but not TBK1 (A). In addition, IRF7, but not IRF3, was precipitated by Ig-MV-VC as efficiently as by full-length Ig-MV-V (B). (C) Native cell lysates of transfected 293T cells were subjected to anti-Flag affinity gel purification and analyzed by Western blotting for interaction of Fl-IKKα, -IRF7, -TBK1, or -IRF3 with Ig-MV-PVN, -MV-PC, or -MV-VC. Ig-MV-VC was coprecipitated efficiently by both IKKα and IRF7. Ig-MV-PVN and Ig-MV-PC did not show interaction with any of the tested proteins. (D) Luciferase reporter gene assay using overexpression of IRF7, MyD88, TRAF6, and IKKα for induction of IFNα6-Luc in 293T cells. In contrast to full-length MV-V, which inhibits induction of the IFNα6 promoter in a dose-dependent manner (250, 500, and 750 ng), Ig-MV-VC or MV-PVN was ineffective. Data are derived from three independent experiments and are represented as means + standard deviations.
Notably, however, in spite of effective binding to IKKα and IRF7, overexpression of Ig-MV-VC did not inhibit stimulation of the IFNα6 promoter, in contrast to full-length Ig-MV-V (Fig. 5D). Thus, while the MV-V C terminus is required and sufficient to bind IKKα and IRF7, the presence of the N terminus in the full-length protein appears to be required in addition for inhibition of IRF7 transcriptional activity.
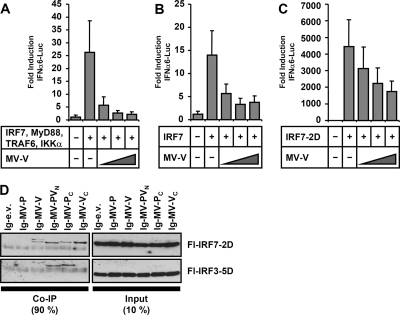
IRF7 is inhibited independent of phosphorylation.
Having identified IKKα and IRF7 as direct targets of MV-V, we addressed the question of at which step IRF7 activity is blocked. We therefore analyzed induction of IFNα6-Luc by overexpression of IRF7, or of IRF7-2D, a constitutively active phosphomimetic IRF7 carrying S477D and S479D mutations (38). A dose-dependent inhibition of FL activity by MV-V was observed in both cases, although the inhibitory effect on IRF7-2D was less pronounced (Fig. 6B and C). The interaction with IRF7-2D was corroborated by coprecipitation of IRF7-2D with both MV-V and the Ig-MV-VC fragment, while IRF3-5D, a constitutively active form of IRF3, was not coprecipitated (Fig. 6D). These results indicated that the interaction of MV-V with IRF7 is independent of the phosphorylation status of IRF7 and that IRF7 may be targeted prior to and postphosphorylation by IKKα.
FIG. 6.
MV-V is able to bind and inhibit activated IRF7. (A to C) Induction of IFNα6 promoter was stimulated in Huh7.5 cells by expression of IRF7 (10 ng plasmid) and MyD88, TRAF6, and IKKα (50 ng each) (A) or by individual expression of IRF7 (100 ng) (B) or constitutively active IRF7-2D (100 ng) (C). Cotransfection of increasing amounts of MV-V (250, 500, 750 ng) revealed a dose-dependent inhibition in all experiments. (D) Plasmids encoding Ig-tagged MV-P, MV-V, MV-PVN, MV-PC, and MV-VC or empty vector were cotransfected with phosphomimetic Fl-IRF7-2D, and Fl-IRF3-5D into 293T cells. Full-length Ig-MV-V as well as Ig-VC was identified to bind IRF7, but not IRF3. Note that precipitated protein bands representing Ig-MV-V, -PVN, and -PC appear in the coimmunoprecipitation (Co-IP) figure (left panel) as well.
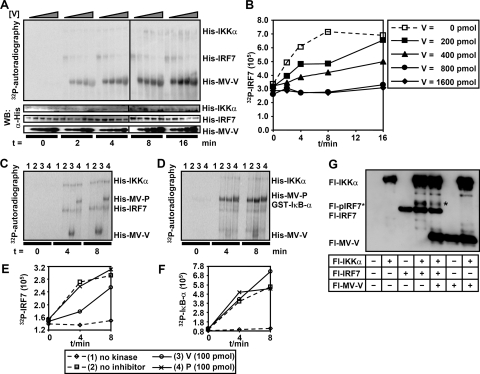
MV-V acts as a decoy substrate for IKKα and blocks phosphorylation of IRF7.
The binding of MV-V to IKKα and IRF7 suggested the possibility that phosphorylation of IRF7 by IKKα is hampered. To address this hypothesis, bacterial expression vectors were constructed and His-MV-V, His-MV-P, and His-IRF7 were expressed in Rosetta (DE3) cells. Proteins purified under native conditions by Ni-NTA column purification were subjected to in vitro kinase assays with commercial recombinant His-IKKα and [γ-32P]ATP. In the presence of constant and equal amounts of His-IRF7 and His-IKKα and increasing amounts of His-MV-V, autoradiography revealed effective labeling of MV-V, identifying the MV-V protein as an excellent substrate for IKKα (Fig. 7A). Moreover, 32P labeling of IRF7 was diminished with increasing amounts of MV-V protein, suggesting successful competition by MV-V protein.
FIG. 7.
MV-V is a substrate of IKKα and competes with IRF7 phosphorylation. His-tagged IRF7, MV-V, and MV-P were expressed in prokaryotic cells and purified by Ni-NTA affinity chromatography under native conditions. (A and B) Equal amounts of IRF7 (500 ng/reaction) and increasing amounts of MV-V (0 to 1,600 pmol per reaction) were subjected to an in vitro kinase assay together with commercial His-IKKα (100 ng/reaction) and [γ-32P]ATP. Probes were subjected to Western blot analysis after incubation at indicated time points. Phosphorylation of proteins was detected by autoradiography, while total protein amounts were determined by Western blotting (WB). (A) MV-V is a substrate for efficient phosphorylation by IKKα in vitro. Increasing amounts of MV-V reduce levels of phospho-IRF7, while total protein levels are equal in all lanes. α-His, anti-His. (B) Quantification of phospho-IRF7 over time with dependence on increasing amounts of MV-V. IRF7 phosphorylation is completely abolished in the presence of large amounts of MV-V (800 and 1,600 pmol), while smaller amounts of MV-V (200 and 400 pmol) delay IRF7 phosphorylation. (C and D) Five hundred nanograms of His-IRF7 (C) or 100 ng of GST-IκB-α (D) was subjected to an in vitro kinase assay in either the absence (lanes 1) or presence (lanes 2 to 4) of 100 ng of His-IKKα and 100 pmol of His-MV-V (lanes 3) or His-MV-P (lanes 4). Reactions were stopped after indicated time points and subjected to Western blot analysis. (E and F) Quantification of phospho-IRF7 (E) or pIκB-α (F) reveals specificity of MV-V. Phosphorylation of IRF7 is inhibited only by MV-V, but not MV-P. Phosphorylation of IκB-α is not inhibited by either viral protein. (G) Reduction of activated IRF7 in living cells. Flag-tagged IKKα, IRF7, and MV-V were pulled down from 293T cells transfected with the indicated plasmids at 24 h posttransfection and analyzed by Western blotting using anti-Flag M2 antibody. A band representing activated IRF7 (marked by a star) appeared only after coexpression of IKKα and IRF7. A significant reduction of this band was observed in the presence of MV-V.
Quantification of phosphorylated IRF7 revealed a dose-dependent reduction of IRF7 phosphorylation at lower doses of MV-V (200 and 400 pmol/reaction), although substantial phosphorylation of IRF7 after a longer incubation time was accomplished. Higher doses (800 and 1,600 pmol/reaction) resulted in an almost complete inhibition of IRF7 phosphorylation (Fig. 7B). In contrast, purified His-MV-P was not able to interfere with phosphorylation of IRF7, although it was also phosphorylated by IKKα (Fig. 7C and E). In contrast to IRF7, phosphorylation of another IKKα substrate, IκB-α, was not inhibited by either MV-V or MV-P. These results indicate that MV-V in vitro acts as a substrate for IKKα which is able to specifically compete with phosphorylation of IRF7.
To verify inhibition of IRF7 phosphorylation in living cells, Fl-IKKα, Fl-IRF7, and Fl-MV-V were expressed in different combinations in 293T cells and pull-down assays were performed using an anti-Flag M2 affinity gel. After coexpression of IRF7 and IKKα, an extra band migrating slower appeared (Fig. 7G) representing activated phospho-IRF7 as it was not observed in cells expressing IRF7 only. The intensity of this band was clearly decreased in the presence of MV-V, while the overall levels of IRF7 were comparable. In summary, the data provide evidence for direct suppression of IRF7 activation by IKKα, which is operational only in the TLR7/9-MyD88-dependent IFN induction.
DISCUSSION
Based on its immunosuppressive features and a Th2-biased immune response (41, 50), we have recently assessed MV for potential effects on pDC. MV was identified to potently inhibit IFN induction in human pDC in vitro in response to both virus infection and exogenous TLR7 and -9 agonists, including R848 and CpG oligodeoxynucleotide (48). Here, we have identified the MV-V protein as instrumental in blocking MyD88-dependent IFNα signaling and have observed an unanticipated mechanism involving both kinase (IKKα) and substrate (IRF7) as binding targets.
Canonical IFN induction following stimulation of the almost ubiquitous cytosolic RNA helicases RIG-I and MDA5, or of TLR3 and TLR4, critically involves the kinases TBK1 and IKKɛ to activate both IRF3 and IRF7. In contrast, MyD88-dependent IFN induction is independent of TBK1/IKKɛ and leads to activation of exclusively IRF7 in a spatiotemporally controlled signaling complex of specialized immune cells equipped with TLR7/8 and -9, such as pDC (30). Here, we exploited transient expression in cell lines of the involved signaling components to mimic MyD88-dependent IRF7 activation and to allow discrimination from TBK1-mediated activation. Specifically, RV-P as an established potent inhibitor of TBK1 and IKKɛ-mediated phosphorylation of IRF3 and IRF7 (4, 6) was not able to interfere with induction of IFNα4 and IFNα6 promoters by the “MyD88 mix,” in contrast to MV-V, while V was not able to block TBK1-mediated IFN induction. The observed specificity of induction also allowed assignment of a critical and dose-dependent role to IKKα as the kinase phosphorylating IRF7, thus corroborating the recently described role of IKKα in TLR7/9-induced IFNα production (29). In contrast, overexpression of IRAK4 or IRAK1, assumed to be responsible for MyD88-dependent IRF7 phosphorylation (58, 59), had no effect or inhibitory effects in 293T or Huh7.5 cells. However, since pDC from IRAK1-deficient mice (59) and humans (60) were found to be severely deficient in the activation of IRF7 and in the production of IFN-α, an essential indirect or regulatory role of this kinase in pDC is suggested.
Intriguingly, both IKKα and IRF7 were found to be high-affinity binding partners of the MV-V protein, but not of MV-P or -C, which are also implicated in MV IFN escape. Significant interaction of MV-V with the closely related kinases IKKβ, TBK1, and IKKɛ or with IRF3 could not be demonstrated, although in individual experiments a residual affinity to TBK1 (Fig. 4C) and IRF3 (Fig. 5C) could not be excluded. MV-V is a multifunctional protein containing an N-terminal 231-residue domain that is shared with the P protein (PVN) and a distinct C-terminal domain of 68 aa (VC) that is cysteine rich and which is conserved among paramyxoviruses. As the MV-P protein failed both in inhibiting MyD88-dependent IFN-α promoter activation and in binding to IRF7 and IKKα, a crucial role of VC was immediately obvious. Actually, an Ig-VC fusion protein was sufficient for binding to both IRF7 and IKKα yet was not active in preventing IFN promoter induction. This suggests that VC is functioning primarily as a targeting module directing full-length V protein to IKKα and IRF7 and indicates a contribution of the common N terminus in the mechanism of inhibition, either by serving as a decoy substrate for IKKα or by more efficiently retaining IRF7 in the cytosol.
As indicated by in vitro kinase experiments, MV-V is a better substrate for IKKα than IRF7 and can successfully compete with IRF7 phosphorylation in vitro, although phosphorylation of IRF7 ensues over time in the presence of V. MV-P was not able to inhibit IRF7 phosphorylation, though it was also used as a substrate for IKKα in vitro. Since V binds IKKα and IRF7 independently from each other, this competing effect can be due to a higher affinity and phosphorylation of V by IKKα or to impaired recruitment of IRF7 to IKKα. Indeed, in transfected cells, the ratio of phosphorylated versus nonphosphorylated IRF7 was found significantly reduced in the presence of MV-V, suggesting an important contribution of these mechanisms to inhibition of IFN induction. Moreover, it is suggested that MV-V may bind in addition to activated IRF7, as indicated by coprecipitation and inhibition of the transcriptional activity of the phosphomimetic IRF7-2D. All of the activities observed are suitable to prevent dimerization and/or import of IRF7 upon TLR7/9 activation into the nucleus.
As for other paramyxovirus V proteins, MV-V, but not MV-P, binds to MDA5 and prevents MDA5 downstream signaling to canonical IFN induction (1, 11). Though rhabdoviruses and paramyxoviruses predominantly activate RIG-I through their 5′-triphosphate leader RNAs (27, 46), a cell-type-dependent contribution by MDA5 may be critical (62). In addition, MV-V counteracts JAK/STAT signaling (7, 43, 44, 55), although the P protein is active in this respect as well (17). In addition, MV-V, but not -P, was reported to copurify with a variety of proteins, including STAT1, STAT2, IRF9, and STAT3 (12, 44), and to block STAT2 phosphorylation (55) and activation of the kinase JAK1 (61). Altogether, these and the present observations prompt the idea of the VC domain as an autonomous and important immune targeting module with multiple distinct destinations.
While it is exciting to see that Paramyxoviridae use their V proteins as a universal tool to simultaneously counteract multiple major pathways of the innate immunity, it is intriguing to see how this tool is adapted by evolution to fit the specific virus need. The uniqueness of the MV-V protein in targeting TLR-MyD88-dependent IFN induction in pDC is emphasized by very recent complementary work. While this article was in preparation, Lu et al. (40) described the inhibition of TBK1/IKKɛ-mediated IRF3 activation by the V proteins of members of the Rubulavirus genus, including mumps virus, human parainfluenza virus 2, and canine parainfluenza virus 5 (previously known as simian virus 5). A direct interaction between V and TBK1/IKKɛ and phosphorylation of V was observed. In contrast, MV-V failed to block TBK1/IKKɛ activity (40), supporting our observation of specific targeting of IKKα. Thus, while rubulaviruses may have the potential to inhibit all but MyD88-dependent IFN induction, the hematopoietic MV may have evolved to specifically target IKKα, probably at the expense of targeting TBK1. Moreover, targeting of IRF7 is a completely novel feature of the MV-V protein.
The ability to simultaneously target IKKα and IRF7 may explain how MV can blindfold pDC and make hosts more permissive to other pathogens. Activation of pDC by a variety of pathogens not only sets off antiviral mechanisms of the type I IFN network but profoundly shapes the host adaptive immune system by promoting cross-presentation and Th1 immune responses (31). Although additional specific mechanisms to counteract adaptive immunity are employed by MV, such as changes in lymphocyte number and function and antigen presentation (51, 52), contact-mediated inhibition of T-cell proliferation (49), and shifts in cytokine responses, particularly of IL-10 and IL-12 (20, 21, 41), it is assumed that the early prevention of pDC function by MV-V protein sets the stage for several of these specific immunosuppressive mechanisms to be more effectual.
A recent study involving V-deficient MV in a rhesus monkey model revealed reduced infectivity of peripheral blood mononuclear cells and lymphatic organs and impaired capacity to control type I IFN and inflammatory cytokines compared to wild-type MV (16). Although pDC are capable of producing IFN independently of the positive IFN feedback loop (2, 32), the JAK/STAT- and MDA5-inhibitory functions of MV-V may contribute to lower systemic IFN levels (26, 57). The results provided here open the way for mutagenesis experiments to generate MV with specific defects in blocking either IKKα, JAK/STAT, or MDA5 signaling, in order to reasonably appreciate the contribution of these individual functions to MV immune biology in vivo. Such studies will not only lead to recombinant vaccine viruses with abolished or reduced capacity to undermine the host immune response but also may help to develop tools to specifically interfere with immunopathological TLR signaling.
Acknowledgments
We thank N. Hagendorf for perfect technical assistance and L. Fragnet for critical reading of the manuscript. MV-P, -V, and -C antisera were kindly provided by R. Cattaneo, D. Gerlier, K. Takeuchi, and S. Schneider-Schaulies, Huh7.5 cells were provided by C. Rice, and cDNAs were provided by S. Akira, J. Hiscott, T. Fujita, K. Ruckdeschel, and A. Kieser.
This work represents part of the Ph.D. thesis of C.K.P. and was supported by the Deutsche Forschungsgemeinschaft through GraKo 1202 “Oligonucleotides in cell biology and therapy” and SFB 455 “Viral functions and immune modulation.”
Footnotes
Published ahead of print on 15 October 2008.
REFERENCES
- 1.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 10117264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellini, W. J., G. Englund, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53908-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brzózka, K., S. Finke, and K.-K. Conzelmann. 2005. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J. Virol. 797673-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brzózka, K., S. Finke, and K. K. Conzelmann. 2006. Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2. J. Virol. 802675-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzózka, K., C. Pfaller, and K. K. Conzelmann. 2007. Signal transduction in the type I interferon system and viral countermeasures. Signal Transduction 715-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caignard, G., M. Guerbois, J. L. Labernardiere, Y. Jacob, L. M. Jones, F. Wild, F. Tangy, and P. O. Vidalain. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 368351-362. [DOI] [PubMed] [Google Scholar]
- 8.Cao, W., and Y. J. Liu. 2007. Innate immune functions of plasmacytoid dendritic cells. Curr. Opin. Immunol. 1924-30. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo, R., K. Kaelin, K. Baczko, and M. A. Billeter. 1989. Measles virus editing provides an additional cysteine-rich protein. Cell 56759-764. [DOI] [PubMed] [Google Scholar]
- 10.Chen, M., J. C. Cortay, and D. Gerlier. 2003. Measles virus protein interactions in yeast: new findings and caveats. Virus Res. 98123-129. [DOI] [PubMed] [Google Scholar]
- 11.Childs, K., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. [DOI] [PubMed] [Google Scholar]
- 12.Cruz, C. D., H. Palosaari, J.-P. Parisien, P. Devaux, R. Cattaneo, T. Ouchi, and C. M. Horvath. 2006. Measles virus V protein inhibits p53 family member p73. J. Virol. 805644-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui, S., K. Eisenacher, A. Kirchhofer, K. Brzózka, A. Lammens, K. Lammens, T. Fujita, K. K. Conzelmann, A. Krug, and K. P. Hopfner. 2008. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol. Cell 29169-179. [DOI] [PubMed] [Google Scholar]
- 14.Delgado, M. A., R. A. Elmaoued, A. S. Davis, G. Kyei, and V. Deretic. 2008. Toll-like receptors control autophagy. EMBO J. 271110-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaux, P., and R. Cattaneo. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J. Virol. 7811632-11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaux, P., G. Hodge, M. B. McChesney, and R. Cattaneo. 2008. Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. J. Virol. 825359-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devaux, P., V. von Messling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 36072-83. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald-Bocarsly, P., and D. Feng. 2007. The role of type I interferon production by dendritic cells in host defense. Biochimie 89843-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin, D. E. 2007. Measles virus, p. 1551-1586. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. Straus (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, PA.
- 20.Griffin, D. E., C. H. Pan, and W. J. Moss. 2008. Measles vaccines. Front. Biosci. 131352-1370. [DOI] [PubMed] [Google Scholar]
- 21.Hahm, B., J. H. Cho, and M. B. Oldstone. 2007. Measles virus-dendritic cell interaction via SLAM inhibits innate immunity: selective signaling through TLR4 but not other TLRs mediates suppression of IL-12 synthesis. Virology 358251-257. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3196-200. [DOI] [PubMed] [Google Scholar]
- 23.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 24.Honda, K., Y. Ohba, H. Yanai, H. Negishi, T. Mizutani, A. Takaoka, C. Taya, and T. Taniguchi. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 4341035-1040. [DOI] [PubMed] [Google Scholar]
- 25.Honda, K., H. Yanai, T. Mizutani, H. Negishi, N. Shimada, N. Suzuki, Y. Ohba, A. Takaoka, W. C. Yeh, and T. Taniguchi. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 10115416-15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434772-777. [DOI] [PubMed] [Google Scholar]
- 27.Hornung, V., J. Ellegast, S. Kim, K. Brzózka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-triphosphate RNA is the ligand for RIG-I. Science 314994-997. [DOI] [PubMed] [Google Scholar]
- 28.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 1684531-4537. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino, K., T. Sugiyama, M. Matsumoto, T. Tanaka, M. Saito, H. Hemmi, O. Ohara, S. Akira, and T. Kaisho. 2006. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature 440949-953. [DOI] [PubMed] [Google Scholar]
- 30.Ishii, K. J., S. Koyama, A. Nakagawa, C. Coban, and S. Akira. 2008. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe 3352-363. [DOI] [PubMed] [Google Scholar]
- 31.Ito, T., R. Amakawa, M. Inaba, T. Hori, M. Ota, K. Nakamura, M. Takebayashi, M. Miyaji, T. Yoshimura, K. Inaba, and S. Fukuhara. 2004. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J. Immunol. 1724253-4259. [DOI] [PubMed] [Google Scholar]
- 32.Izaguirre, A., B. J. Barnes, S. Amrute, W. S. Yeow, N. Megjugorac, J. Dai, D. Feng, E. Chung, P. M. Pitha, and P. Fitzgerald-Bocarsly. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 741125-1138. [DOI] [PubMed] [Google Scholar]
- 33.Kawai, T., S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 51061-1068. [DOI] [PubMed] [Google Scholar]
- 34.Kim, T. W., K. Staschke, K. Bulek, J. Yao, K. Peters, K. H. Oh, Y. Vandenburg, H. Xiao, W. Qian, T. Hamilton, B. Min, G. Sen, R. Gilmour, and X. Li. 2007. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 2041025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krieg, A. M., and J. Vollmer. 2007. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol. Rev. 220251-269. [DOI] [PubMed] [Google Scholar]
- 36.Krug, A. 2008. Nucleic acid recognition receptors in autoimmunity. Handb. Exp. Pharmacol. 183129-151. [DOI] [PubMed] [Google Scholar]
- 37.Lee, H. K., J. M. Lund, B. Ramanathan, N. Mizushima, and A. Iwasaki. 2007. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 3151398-1401. [DOI] [PubMed] [Google Scholar]
- 38.Lin, R., Y. Mamane, and J. Hiscott. 2000. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem. 27534320-34327. [DOI] [PubMed] [Google Scholar]
- 39.Liston, P., and D. J. Briedis. 1994. Measles virus V protein binds zinc. Virology 198399-404. [DOI] [PubMed] [Google Scholar]
- 40.Lu, L. L., M. Puri, C. M. Horvath, and G. C. Sen. 2008. Select paramyxoviral V proteins inhibit IRF3 activation by acting as alternative substrates for IKKE/TBK1. J. Biol. Chem. 28314269-14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss, W. J., M. O. Ota, and D. E. Griffin. 2004. Measles: immune suppression and immune responses. Int. J. Biochem. Cell Biol. 361380-1385. [DOI] [PubMed] [Google Scholar]
- 42.Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J. Virol. 8011861-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 852991-2999. [DOI] [PubMed] [Google Scholar]
- 44.Palosaari, H., J.-P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 777635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paterson, R. G., G. P. Leser, M. A. Shaughnessy, and R. A. Lamb. 1995. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology 208121-131. [DOI] [PubMed] [Google Scholar]
- 46.Plumet, S., F. Herschke, J. M. Bourhis, H. Valentin, S. Longhi, and D. Gerlier. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE 2e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao, N., S. Nguyen, K. Ngo, and W.-P. Fung-Leung. 2005. A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in Toll/IL-1R-induced inflammatory signaling. Mol. Cell. Biol. 256521-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlender, J., V. Hornung, S. Finke, M. Günthner-Biller, S. Marozin, K. Brzózka, S. Moghim, S. Endres, G. Hartmann, and K.-K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 795507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlender, J., J. J. Schnorr, P. Spielhoffer, T. Cathomen, R. Cattaneo, M. A. Billeter, V. ter Meulen, and S. Schneider-Schaulies. 1996. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc. Natl. Acad. Sci. USA 9313194-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider-Schaulies, S., I. M. Klagge, and V. ter Meulen. 2003. Dendritic cells and measles virus infection. Curr. Top. Microbiol. Immunol. 27677-101. [DOI] [PubMed] [Google Scholar]
- 51.Servet-Delprat, C., P.-O. Vidalain, O. Azocar, F. Le Deist, A. Fischer, and C. Rabourdin-Combe. 2000. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J. Virol. 744387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Servet-Delprat, C., P. O. Vidalain, H. Bausinger, S. Manie, F. Le Deist, O. Azocar, D. Hanau, A. Fischer, and C. Rabourdin-Combe. 2000. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J. Immunol. 1641753-1760. [DOI] [PubMed] [Google Scholar]
- 53.Shaffer, J. A., W. J. Bellini, and P. A. Rota. 2003. The C protein of measles virus inhibits the type I interferon response. Virology 315389-397. [DOI] [PubMed] [Google Scholar]
- 54.Sumpter, R., Jr., Y.-M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 792689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeuchi, K., S. I. Kadota, M. Takeda, N. Miyajima, and K. Nagata. 2003. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545177-182. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi, K., M. Takeda, N. Miyajima, Y. Ami, N. Nagata, Y. Suzaki, J. Shahnewaz, S.-I. Kadota, and K. Nagata. 2005. Stringent requirement for the C protein of wild-type measles virus for growth both in vitro and in macaques. J. Virol. 797838-7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamura, T., H. Yanai, D. Savitsky, and T. Taniguchi. 2008. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26535-584. [DOI] [PubMed] [Google Scholar]
- 58.Uematsu, S., and S. Akira. 2007. Toll-like receptors and type I interferons. J. Biol. Chem. 28215319-15323. [DOI] [PubMed] [Google Scholar]
- 59.Uematsu, S., S. Sato, M. Yamamoto, T. Hirotani, H. Kato, F. Takeshita, M. Matsuda, C. Coban, K. J. Ishii, T. Kawai, O. Takeuchi, and S. Akira. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J. Exp. Med. 201915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, K., A. Puel, S. Zhang, C. Eidenschenk, C. L. Ku, A. Casrouge, C. Picard, H. von Bernuth, B. Senechal, S. Plancoulaine, S. Al Hajjar, A. Al Ghonaium, L. Marodi, D. Davidson, D. Speert, C. Roifman, B. Z. Garty, A. Ozinsky, F. J. Barrat, R. L. Coffman, R. L. Miller, X. Li, P. Lebon, C. Rodriguez-Gallego, H. Chapel, F. Geissmann, E. Jouanguy, and J. L. Casanova. 2005. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity 23465-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokota, S., H. Saito, T. Kubota, N. Yokosawa, K. Amano, and N. Fujii. 2003. Measles virus suppresses interferon-alpha signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-alpha receptor complex. Virology 306135-146. [DOI] [PubMed] [Google Scholar]
- 62.Yount, J. S., L. Gitlin, T. M. Moran, and C. B. Lopez. 2008. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai virus defective interfering particles. J. Immunol. 1804910-4918. [DOI] [PubMed] [Google Scholar]