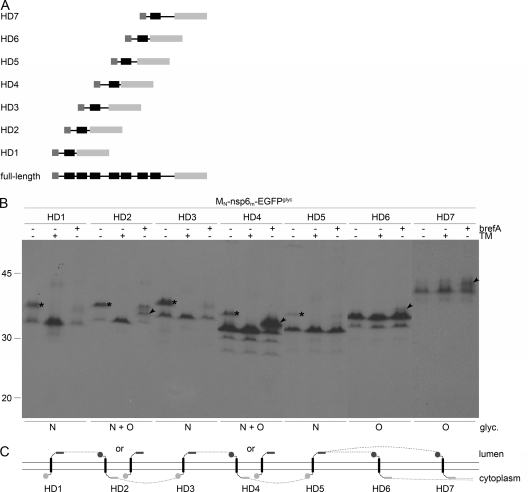
FIG. 7.
Membrane integration of MHV nsp6 hydrophobic domains. (A) Schematic representation of the MHV nsp6 constructs. (B) vTF7-3-infected OST7-1 cells were transfected with the constructs presented in panel A and expressed in the presence (+) or absence (−) of tunicamycin (TM) or brefeldin A (brefA). The cells were labeled with [35S]methionine from 5 to 6 h p.i., lysed, and processed for immunoprecipitation with anti-EGFP antiserum, followed by SDS-PAGE. The positions and masses (in kilodaltons) of the protein size markers are indicated at the left. Only the relevant portion of the gel is shown. The asterisks indicate the position of the N-glycosylated protein species, and the arrowhead indicates that of the O-glycosylated protein species. Below the panels, the observed presence or absence of N- and or O-linked oligosaccharides is indicated. (C) Model of the membrane topologies of the nsp6 hydrophobic domains. The hydrophobic domains are presented as black rectangles, the EGFPglyc tag is presented as a gray rectangle, and the MN tag is presented as a gray circle. Dark gray rectangles or circles represent glycosylated tags, and light gray rectangles or circles represent unglycosylated tags. The dotted line connects the transmembrane domains in accordance with the full-length nsp6 structure, indicating that either the sixth or the seventh hydrophobic domain does not traverse the lipid bilayer.