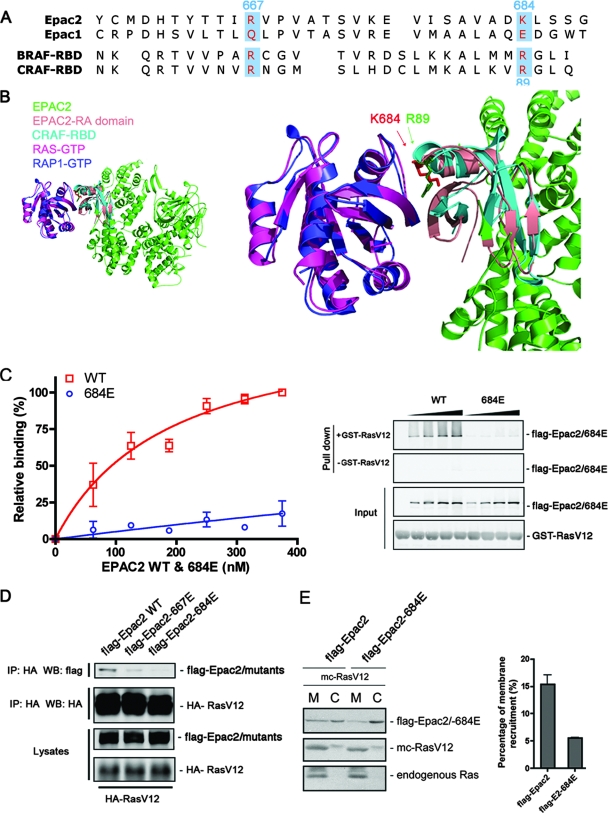
FIG. 2.
Disruption of Ras-Epac2 interaction by a single point mutation within the RA domain. (A) Sequence alignment of the RA domains from Epac1 and Epac2, as well as the RBDs from B-Raf (BRAF-RBD) and C-Raf (CRAF-RBD). The residues within Epac2 that were targeted for mutational analysis are highlighted. (B) Structural modeling demonstrating the position of K684 at the Ras-Epac2 binding interface. The crystal structure of Rap1 in a complex with the C-Raf RBD was used as the template, and the structures of Ras and Epac2 were superimposed onto Rap1 and the C-Raf RBD, respectively, with the program Chimera (University of California at San Francisco). The left panel shows an overview of the predicted Ras-Epac2 complex; the right panel shows a close-up of the binding interface. The side chains of K684 (Epac2) and R89 (C-Raf) are highlighted as red and green sticks, respectively (arrows). (C) Epac2-684E was incapable of Ras binding. Increasing amounts of wild-type (WT) Flag-Epac2 or Epac2-684E were incubated with GTPγS-loaded GST-RasV12, followed by a GST pull-down assay and Western blotting. The concentration of Flag-tagged protein was quantified by comparison to purified protein standards. The graph shows data from three experiments (mean ± standard error) with curve fitting. The right panel shows the amounts of Epac proteins pulled down in the presence or absence of GST-RasV12, as well as the input levels of all of the proteins. (D) Ras association with wild-type Epac2 and Epac2 mutant forms. Wild-type Flag-Epac2 and mutant Epac2-667E or Epac2-684E were cotransfected with HA-RasV12 into COS cells, and immunoprecipitation (IP) was performed with anti-HA agarose antibody, followed by Western blotting (WB). The first two rows show the levels of Epac2 proteins and HA-RasV12 immunoprecipitated. The third and fourth rows show the levels of transfected proteins within the lysates. (E) The recruitment of Epac2-684E to the membrane is reduced compared to that of wild-type Epac2. Wild-type Flag-Epac2 or Epac2-684E was cotransfected with mCherry (mc)-RasV12 into COS cells, followed by cell fractionation. The membrane fraction (M) and the cytosolic fraction (C) were isolated and examined by Western blotting with Flag antibody (top). Both transfected RasV12 (mc-RasV12; middle) and endogenous Ras (bottom) were also monitored as markers for the membrane fraction with Ras antibody. The percentages of wild-type Epac2 and mutant Epac2-684E that were recruited to the membrane were calculated as described in Materials and Methods, and the right panel summarizes data from three experiments (mean ± standard error).