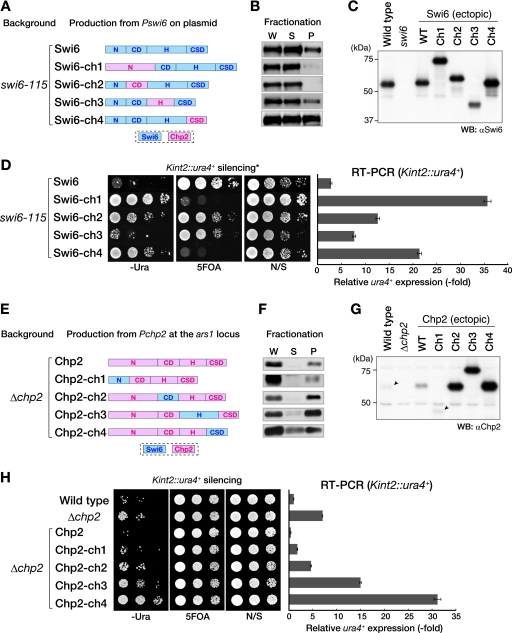
FIG. 8.
Silencing functions of Swi6 and Chp2 are linked with the property of chromatin/nuclear association. (A to D) Swi6 function is correlated with its chromatin association state. (A) Using the alignment of conserved regions (see Fig. S1 in the supplemental material), the Swi6 and Chp2 proteins were divided into four domains: the N terminus (aa 1 to 66 of Swi6; aa 1 to 160 of Chp2), CD (aa 66 to 133 of Swi6; aa 159 to 230 of Chp2), H region (aa 134 to 261 of Swi6; aa 229 to 316 of Chp2), and CSD (aa 262 to 328 of Swi6; aa 315 to 380 of Chp2). Chimeric Swi6 proteins containing Chp2N, Chp2CD, Chp2H, or Chp2CSD were produced from the swi6 promoter on an episomal plasmid (pRE) in a swi6-115 mutant background. Expressed chimeric proteins were detected by Western blotting using anti-Swi6 antibody (C) and were subjected to the chromatin fractionation assay (B). The silencing of Kint2::ura4+ in swi6-115 mutant cells was evaluated by spotting assay and RT-PCR (D). Domains derived from Swi6 and Chp2 are indicated by blue and pink boxes, respectively. (E to H) Chp2 function is correlated with its chromatin-associated state. Chimeric Chp2 protein containing Swi6N, Swi6CD, Swi6H, or Swi6CSD was produced from the chp2+ promoter at the ars1 locus (E). Expressed chimeric proteins were detected by Western blotting using an anti-Chp2 antibody (G) and were subjected to the chromatin fractionation assay (F). W, whole-cell extract; S, supernatant fraction; P, pellet fraction. Arrowheads indicate the positions of endogenous Chp2 in wild-type cells and ectopically expressed Chp2-ch1, respectively (G). The silencing of Kint2::ura4+ in Δchp2 cells was also evaluated by spotting assay and RT-PCR (H). α, anti; WT, wild type; N/S, nonselective medium.