Fig. 5.
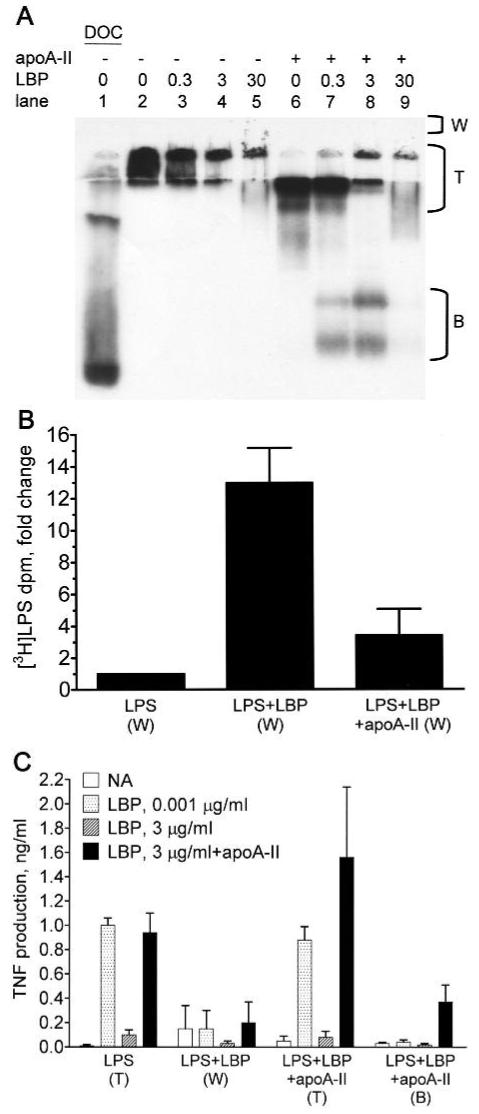
ApoA-II prevents the formation of large LPS-LBP complexes that inhibit LPS bioactivity. [3H]-LPS (0.1 μg) was incubated for 30 min at 37°C in 7.5 μl of SFM without BSA containing the indicated concentrations (μg/ml) of LBP in the presence or absence of 1.4 μg of apoA-II (apoA-II:LPS molar ratio = 4). (A) The mixtures were run on a 10% native gel and subjected to fluorography to detect the [3H]-LPS. Region (T) includes material near the top of the running gel and the stacking gel. (B) The indicated mixtures of [3H]-LPS alone (LPS), [3H]-LPS and LBP (3 μg/ml; LPS+LBP), and [3H]-LPS, LBP, and apoA-II (LPS+LBP+apoA-II) were run on separate gels as described above. Material that did not enter the gel was rinsed out of the wells above the stacking gel (region ‘W’ in panel A) and counted for recovery of [3H]-LPS. The recovered tritium dpm were 895 ± 236 (LPS), 11,951 ± 5296 (LPS+LBP), and 2980 ± 1287 (LPS+LBP+apoA-II; mean ± SD; n = 3 experiments). The data were normalized and expressed as the fold-change from LPS alone. Error bars denote mean ± SD. (C) To determine bioactivity of the LPS recovered from the indicated regions of gels, the LPS concentration was determined by counting tritium dpm, and 100 pg/ml of LPS was incubated with THP-1 cells in the presence of the indicated concentrations of LBP and apoA-II (100 μg/ml) as described in the caption to Figure 1. Error bars denote mean ± SD (n = 5-8) of duplicate determinations in 3 or 4 experiments.
