Abstract
Epidemiological evidence indicates that prolonged lifetime exposure to estrogen is associated with elevated breast cancer risk in women. Oxidative stress and estrogen receptor-associated proliferative changes are suggested to play important roles in estrogen-induced breast carcinogenesis. In the present study, we investigated changes in breast morphology and oxidative stress following estrogen exposure. Female ACI rats were treated with 17β-estradiol (E2, 3 mg, s.c.) for either 7, 15, 120 or 240 days. Animals were sacrificed, tissues were excised, and portions of the tissues were either fixed in 10% buffered formalin or snap-frozen in liquid nitrogen. Paraffin-embedded tissues were examined for histopathologic changes. Proliferative changes appeared in the breast after 7 days of E2 exposure. Atypical ductal proliferation and significant reduction in stromal fat were observed following 120 days of E2 exposure. Both in situ and invasive carcinomas were observed in the majority of the mammary glands from rats treated with E2 for 240 days. Palpable breast tumors were observed in 82% of E2-treated rats after 228 days, with the first palpable tumor appearing after 128 days. No morphological changes were observed in the livers, kidneys, lungs or brains of rats treated with E2 for 240 days compared to controls. Furthermore, 8-isoprostane (8-isoPGF2α) levels as well as the activities of antioxidant enzymes, such as glutathione peroxidase, superoxide dismutase and catalase, were quantified in the breast tissues of rats treated with E2 for 7, 15, 120 and 240 days and compared to activity levels in age-matched controls. 8-isoPGF2α levels displayed time-dependent increases upon E2 treatment and were significantly higher than control levels at the 15, 120 and 240 day time-points. 8-isoPGF2α levels observed in E2-induced mammary tumors were significantly higher than levels found in control mammary tissue from age-matched animals. Similarly, alterations in glutathione peroxidase and superoxide dismutase activities were detected in both mammary and tumor tissue from E2-treated rats. Taken together, our data reveal that proliferative changes in the breast tissue of ACI rats are associated with increases in 8-isoPGF2α formation as well as changes in the activities of antioxidant enzymes. These oxidative changes appear to be a function of E2 exposure and occur prior to tumor development.
Keywords: Breast cancer, ACI rat, animal model of breast cancer, estrogen, 17β-estradiol, histopathology, mammary tumor, oxidative stress
Introduction
In the United States, breast cancer represents the most common neoplasm and the second most frequent cause of cancer death in women (Galati and O'Brien, 2004; Ju et al., 2006). Since 2003, the incidence of breast cancer in the United States has decreased, possibly reflecting reduced utilization of hormone replacement therapy (Ries et al., 2007). In contrast, global registry data demonstrates that breast cancer incidence has been increasing in most parts of the world since 1973 (IARC, 2007). The importance of estrogens in the etiology of breast cancer is widely recognized and the United States government has added steroidal estrogens to the list of known human carcinogens (IARC, 1987; Cavalieri et al., 1997; IARC, 1999; NTP, 2002; Bhat et al., 2003; Cavalieri et al., 2006). Elevated lifetime estrogen exposure is considered a major risk factor for breast cancer, and many known risk factors for the disease are related to a woman's reproductive history (McPherson et al., 2000; Nelson et al., 2002; Rossouw et al., 2002). For example, breast cancer risk factors include early menarche, nulliparity or late pregnancy, late menopause, prolonged oral contraceptive use and hormone replacement therapy (McPherson et al., 2000; Nelson et al., 2002; Rossouw et al., 2002). While a compelling body of data supports a role for estrogen in breast carcinogenesis, the molecular mechanisms underlying the initiation and progression of estrogen-related cancers remain indefinite (Clemons and Goss, 2001; Key et al., 2002).
Estrogens exert their carcinogenic effects by both estrogen receptor (ER)-dependent and ER-independent mechanisms (Liehr et al., 1993; Clemons and Goss, 2001; Bhat et al., 2003; Yager and Davidson, 2006). The ER-dependent pathway involves the activation of the ER by estrogens, leading to the expression of estrogen-responsive genes, as well as stimulation of cell growth and proliferation (Liehr et al., 1993; Clemons and Goss, 2001; Bhat et al., 2003; Cavalieri et al., 2006; Yager and Davidson, 2006). Moreover, estrogens promote the expansion of estrogen-responsive neoplastic cells, resulting in accumulation of genomic damage (Liehr et al., 1993; Clemons and Goss, 2001; Bhat et al., 2003; Cavalieri et al., 2006; Yager and Davidson, 2006). The ER-independent pathway of estrogen-induced breast cancer involves the generation of genotoxic estrogen metabolites, which are highly reactive and damage DNA (Clemons and Goss, 2001; Patel and Bhat, 2004; Yager and Davidson, 2006). 17β-estradiol (E2) is metabolized to catechol estrogens 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2) by cytochrome P450 1A1 (Cyp1A1) and cytochrome P450 1B1 (Cyp1B1), respectively (Cavalieri et al., 1997; Cavalieri and Rogan, 2004). While, 2-OHE2 and 2-methoxyestradiol (2-MeOE2) have putative chemo-protective characteristics, 4-OHE2 is highly genotoxic (Liehr et al., 1986; Li and Li, 1987; Ziegler et al., 1997; Badawi et al., 2001; Brueggemeier et al., 2001; LaVallee et al., 2002). Tumorigenic estrogen metabolites such as 4-OHE2 undergo oxidative metabolism to form electrophilic quinones, which readily react with DNA to produce depurinating adducts (Liehr et al., 1993; Cavalieri et al., 1997; Cao et al., 1998; Patel and Bhat, 2004). In addition, redox cycling of quinones and semiquinones results in the formation of free radicals and reactive oxygen species, thereby creating more opportunities for genetic damage (Liehr et al., 1986; Yager, 2000). Recent studies also suggest that mitochondria may also be involved in the generation of estrogen-associated reactive oxygen species (ROS) (Felty et al., 2005a; Felty et al., 2005b; Roy et al., 2007). The oxidative stress generated by estrogens is suspected to act in concert with ER-mediated signaling pathways to promote DNA damage and altered expression of genes responsible for controlling cell cycle and proliferation (Liehr et al., 1986; Cavalieri et al., 1997; Yager, 2000; Bhat et al., 2003; Cavalieri and Rogan, 2004).
The ACI rat model of estrogen-induced mammary cancer provides a relevant biological system in which to study the molecular mechanisms underlying human breast cancer. Continuous treatment of ovary-intact female ACI rats with E2 results in nearly 100% mammary tumor incidence within six to seven months (Shull et al., 1997; Harvell et al., 2000). Mammary tumors have not been observed in ovary-intact female ACI rats not exposed to exogenous E2 (Shull et al., 1997; Harvell et al., 2000). All mammary tumors observed in this model have been classified as carcinomas, and invasive features have been observed in some (Shull et al., 1997; Harvell et al., 2000). E2-induced mammary gland tumors in ACI rats and human sporadic breast cancers share many pertinent histopathologic and molecular features, both in early pre-malignant lesions as well as in primary tumors (Makris et al., 1997; Arnerlov et al., 2001; Li et al., 2002a; Li et al., 2002b; Li et al., 2004; Weroha et al., 2006). For example, mammary cancers induced by E2 in ACI rats are estrogen-dependent and exhibit genomic instability, characteristics commonly observed in human breast cancers (Makris et al., 1997; Arnerlov et al., 2001; Li et al., 2002a; Li et al., 2002b; Li et al., 2004; Weroha et al., 2006).
The objective of the current study was to investigate the occurrence of changes in breast morphology and oxidative stress following estrogen exposure in vivo using the ACI rat model of breast carcinogenesis. Female ACI rats were treated with E2 (3 mg, s.c.) for 7, 15, 120 and 240 days. Animals were examined daily for palpable tumors. At the end of the experiments, animals were sacrificed and various tissues, including breast (an estrogen-target tissue) and liver (a non-target tissue), were excised and examined for changes in levels of oxidative stress markers and histopathologic alterations. Our findings indicate that proliferative changes in the breast tissue of E2-stimulated rats are associated with increases in 8-isoPGF2α formation and alterations in the activities of antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx). Thus, changes in oxidative stress markers seem to be a function of duration of exposure to E2 and precede tumor development.
Materials and Methods
Tumor development/estrogen treatment of rats and histopathologic analysis
Female ACI rats, 4 weeks of age (Harlan Sprague Dawley, Indianapolis, IN) were housed in the Columbia University animal facility under controlled temperature, humidity, and lighting conditions. The animals were fed an AIN-76A phytoestrogen-free diet (Dyets, Bethlehem PA) and water was given ad libitum. Animals were randomly distributed into two groups—control and E2-treatment. After a one-week acclimatization period, rats in the E2-treatment group were implanted subcutaneously with E2 pellets (3 mg E2 + 17 mg cholesterol) and rats in the control group were implanted with cholesterol pellets (17 mg cholesterol). E2 and cholesterol pellets were prepared using a pellet press as described previously (Han and Liehr, 1994; Wang and Liehr, 1995; Bhat et al., 2003). Eight separate sub-groups of rats, containing at least 10 rats per group, were treated with either E2 or cholesterol pellets for 7, 15, 120 or 240 days. At the end of each of these time periods, animals were anesthetized using isoflurane and sacrificed. Mammary tissues (both tumor and normal) as well as liver, uterus, kidney, lung and brain tissues were removed and snap-frozen in liquid nitrogen for future analyses. Frozen tissues were stored at −70°C. A portion of the excised tissues was stored in 10% buffered formalin for histopathologic and immunohistochemical analyses. Tumor incidence and the number of tumor nodules were counted at the time of dissection. The formalin-fixed tissue was embedded in paraffin, and sections of 4- to 5-μm thickness were cut. Paraffin-embedded sections of the mammary, liver, brain, uterus, kidney, lung and ovary were stained with hematoxylin and eosin for histopathologic evaluation by a pathologist. Tumors in the breast fat pads were identified grossly at the time of animal dissection. Tumor size was measured in millimeters and the volume (length × breadth × height) of tumor in cubic millimeters was obtained.
Determination of 8-iso-Prostane F2α(8-isoPGF2α)
Total 8-iso-Prostane F2α(8-isoPGF2α) levels in the mammary and liver tissue of female ACI rats were quantified using a direct 8-isoPGF2α enzyme immunoassay kit obtained from Assay Designs (Ann Arbor, MI) according to the suppliers instructions as described previously (Bhat et al., 2003). Fifty to 100 mg rat liver or mammary tissue was homogenized in cold PBS (pH 7.4) containing 0.005% butylated hydroxytolulene. Tissue homogenates were prepared in 2 ml round-bottom microcentrifuge tubes by using a TissueLyser (Qiagen, Valencia CA). Homogenization was carried out in the TissueLyser at 29 cycles per second for 4 minutes. Protein concentrations from the neutralized homogenates were determined using the Pierce BCA protein assay kit (Pierce, Rockford IL). 8-isoPGF2α esters in 100 μl of the total liver or mammary homogenate were hydrolyzed by incubation with 25 μ of 10 N NaOH at 45°C for 2 hours. The reaction mixture was cooled on ice for 5 minutes, neutralized with 25 μl of 12 N HCl and centrifuged in a microcentrifuge for 5 minutes 4°C. The clear neutralized supernatant was transferred into a new microcentrifuge tube and 50 μl of the hydrolyzed/neutralized sample was used for the 96-well format 8-isoPGF2α assay. Samples were incubated with the 8-isoPGF2α antibody for 18 hours at 4°C. After incubation, the contents of the wells were emptied and washed with wash buffer. Wash buffer was then removed from the wells and the color was developed by incubation with 200 μl of p-nitrophenyl phosphate for 45 minutes at room temperature. The reaction was stopped by the addition of 50 μl of stop solution and the plate was read at 405 nm. Standard curves were run on each plate and were generated by measuring the optical density of 160-100,000 pg/ml of 8-isoPGF2α standards that were processed simultaneously with the unknown samples. Data are expressed as mean 8-isoPGF2α pg/mg protein ± standard error of the mean. Fold changes were calculated by comparing 8-isoPGF2α levels detected in the mammary or liver tissue of E2-exposed animal tissues to levels in the mammary or liver tissue of age-matched control animals.
Measurement of superoxide dismutase (SOD) activity
SOD activity in the mammary tissue of ACI rats was measured using a commerically-available kit from Cayman Chemical Company (Catalog Number 706002, Ann Arbor MI). Briefly, SOD activity was quantified using a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. At the time of dissection, liver and mammary tissues were rinsed in PBS (pH 7.4) in order to remove red blood cells and clots. Tissues were subsequently snap-frozen in liquid nitrogen and stored at −80°C. At the time of analysis, approximately 50 mg of mammary or liver tissue was homogenized in 50 mM phosphate buffer containing 1 mM EDTA, 210 mM mannitol and 70 mM sucrose. Homogenization was performed using a TissueLyser (Qiagen, Valencia CA) for 4 minutes with the frequency set to 30 cycles per second. After homogenization, homogenates were centrifuged at 1500 × g for 5 minutes at 4°C. The supernatant was removed and the protein concentration was measured using the BCA Protein Assay (Pierce, Rockford IL). Prior to measurement of SOD activity, mammary tissue homogenates were diluted with phosphate buffer to a concentration of 85 μg/ml. Liver tissue homogenates were diluted to a concentration of 8.5 μg/ml. Optimization of the assay showed these concentrations to produce absorbances within the linear range of the standard curve. Analysis of SOD activity in the tissue homogenates was carried out according to the manufacturer's instructions in 96-well format. Standard curves were run on each plate and were generated by measuring the optical density of seven samples with SOD activity ranging from 0-0.25 units/ml. The absorbance of the sample and standard wells were measured at 405 nm using a plate reader. SOD activity is reported as units/mg protein. One unit of SOD is defined as the amount of enzyme needed to cause 50% dismutation of the superoxide radical. Fold changes in SOD activity were calculated by comparing SOD activity in the mammary or liver tissue of E2-treated ACI rats to SOD activity measured in the mammary or liver tissue of age-matched controls.
Quantification of catalase (CAT) activity
CAT activity in the mammary tissue of ACI rats was quantified using a kit commercially-available from Cayman Chemical Company (Catalog Number 707002, Ann Arbor MI). This method is based on the reaction of the CAT enzyme with methanol in the presence of an optimal concentration of H2O2. The formaldehyde produced was measured spectrophotometrically at 540 nm using 4-amino-3-hydrazino-5-mercapto-1, 2, 4-triazole (Purpald) as the chromogen. Purpald specifically forms a bicyclic heterocycle with aldehydes, which changes from colorless to purple following oxidation. At the time of analysis, approximately 50 mg of mammary or liver tissue was homogenized in 50 mM phosphate buffer containing 1 mM EDTA, 210 mM mannitol and 70 mM sucrose. Homogenization was carried out as described above. After homogenization, homogenates were centrifuged at 10,000 × g for 15 minutes at 4°C. The supernatant was removed and the protein concentration was measured as described above. Prior to measurement of CAT activity, mammary tissue homogenates were diluted with phosphate buffer to a concentration of 75 μg/ml. Liver homogenates were diluted to a concentration of 8.5 μg/ml. Optimization of the assay showed these concentrations to produce absorbances within the linear range of the standard curve. Analysis of CAT activity in the tissue homogenates was performed according to the manufacturer's instructions in 96-well format. Standard curves were run on each plate and were generated by measuring the optical density of seven standards. CAT activity is reported as nmol/min/mg protein. Fold changes in CAT activity in E2-treated rats were calculated by comparing CAT activity in the mammary or liver tissue of E2-treated rats to that of mammary or liver tissue from age-matched controls.
Determination of glutathione peroxidase (GPx) activity
GPx activity in the mammary tissue of ACI rats was determined by using a kit available from Cayman Chemical Company (Catalog Number 703102, Ann Arbor MI). The GPx Assay kit measures GPx activity by way of a coupled reaction with glutathione reductase. Oxidized glutathione is produced during reduction of an organic hydroperoxide by GPx, and is recycled to its reduced state by glutathione reductase and NADPH. The oxidation of NADPH to NADP+ causes a decrease in absorbance at 340 nm. The rate of decrease in the absorbance of the samples at 340 nm is directly proportional to the GPx activity in the sample. At the time of analysis, approximately 50 mg of mammary or liver tissue was homogenized in 50 mM phosphate buffer containing 1 mM EDTA, 210 mM mannitol and 70 mM sucrose. Homogenization was carried out as described above. After homogenization, homogenates were centrifuged at 10,000 × g for 15 minutes at 4°C. The supernatant was removed and the protein concentration was measured as described above. Prior to measurement of GPx activity, mammary tissue homogenates were diluted with phosphate buffer to a concentration of 150 μg/ml. Liver homogenates were diluted to a concentration of 8.5 μg/ml. Optimization of the assay showed these concentrations to produce absorbances within the linear range of the assay. Analysis of GPx activity in the tissue homogenates was carried out according to the manufacturer's instructions in 96-well format. Seven time points, spanning 7 minutes, were obtained in order to accurately assess the decrease in absorbance at 340 nm. GPx activity was reported as nmol/min/mg protein. Fold changes in GPx activity were calculated by comparing GPx activity in the mammary or liver tissue of E2-treated ACI rats to that of mammary or liver tissue from age-matched control rats.
Statistical Analyses
All data were analyzed using Sigma Plot 8.0 (Systat Software, San Jose CA). Tumor incidence was calculated by dividing the number of animals with tumors by the total number of animals in the experimental group. The average number of tumor nodules per tumor-bearing animal was calculated by dividing the sum of the tumor nodules in all tumor-bearing animals by the total number of tumor-bearing animals. The average number of tumor nodules per rat is expressed as the mean ± the standard error. Average tumor size was calculated as the mean of the tumor sizes present in tumor-bearing animals and is expressed as mean ± standard error. 8-isoPGF2α, SOD, GPx and CAT assays were all performed in duplicate using samples from at least 7 different animals in each of the treatment groups. The unpaired t-test analysis was used to calculate p values for comparisons of 8-iso-PGF2α levels, SOD, GPx and CAT activity between estrogen-treated rats and age-matched controls. P values < 0.05 were considered significant.
Results
Mammary tissue from female ACI rats displayed morphological changes following E2 exposure
A number of morphological changes were observed in the mammary tissue of rats treated with E2. Mammary tissue from 100% of rats exposed to E2 for 7 days revealed hyperplastic lobular units that expanded into the stromal fat (data not shown). Progressive increases in lobular hyperplasia, elongation of ducts and compression of stromal fat were observed in the mammary tissue of all animals treated for 15 days with E2 (Figure 1). In contrast, mammary tissue from 100% age-matched cholesterol-treated control rats showed normal tubulolobular architecture, consisting of branched ducts terminating in alveolar buds (Figure 1). The branched tubuloalveolar units are evenly distributed in the abundant fat pad of the mammary gland (Figure 1). While proliferative changes in mammary tissue following prolonged exposure to E2 have been previously reported, early changes, such as those present after 7 or 15 days E2 exposure, have not yet been reported in the literature. Further expansion of the hyperplastic lobules and significant reduction in stromal fat was observed in mammary tissue from 100% of rats treated with E2 for 120 days (data not shown). In addition to hypertrophic and hyperplastic changes, E2 treatment resulted in the appearance of malignant lesions. Cystic papillary carcinoma was observed in the mammary tissue from ∼8% of rats after 120 days E2 exposure (Figure 2). Mammary tissue from 100% of control rats remained unchanged after 120 days and was morphologically similar to tissue from animals in the 7- and 15-day control groups (data not shown). After 240 days treatment with E2, mammary tissue from 100% of the rats in the E2 treatment group showed advanced expansion of hyperplastic lobules, dilated ducts containing inspissated secretions and further compression of stromal fat (Figure 3). In situ carcinomas were present in mammary glands from ∼80% of rats treated with E2 for 240 days, and invasive carcinomas were present in mammary glands from ∼40% of rats treated with E2 for 240 days (Figure 3). No morphological changes were observed in the liver, kidney, lung or brain tissues of any of the E2-treated rats compared to age-matched control rats (data not shown). Approximately 9% of E2-treated animals displayed squamous metaplasia of the uteri (data not shown). No vasculo-lymphatic invasion, mammary lymph node involvement, or distant organ metastases (lung, liver) were identified in any of the tissues examined.
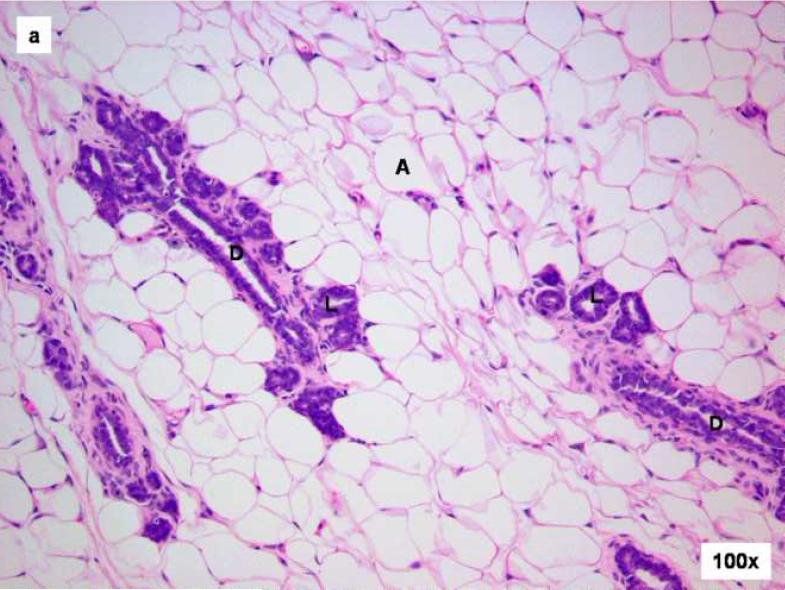
Figure 1. Comparison of control and E2-treated mammary gland after 15 days.
Female ACI rats were treated with an E2 pellet (s.c., 3 mg E2 + 17 mg cholesterol) for 15 days. Control rats were implanted with a pellet containing 17 mg cholesterol only. Histopathologic analyses were performed on mammary tissue from at least 4 control and 4 E2-treated animals. This figure shows mammary tissue representative of the morphology generally observed in control and E2-treated animals. a) The mammary gland of a control ACI rat shows normal tubulolobular architecture (L), consisting of branched ducts (D) terminating in alveolar buds. The branched tubuloalveolar units are more or less evenly distributed in the abundant fat pad (A) of the mammary gland. b) The mammary glands of a rat treated with E2 for 15 days showed proliferative changes characterized by a progressive time-dependent elongation of ducts (D) as well as proliferation and expansion of the terminal lobular units (HLU) with compression of the surrounding fat pad (A).
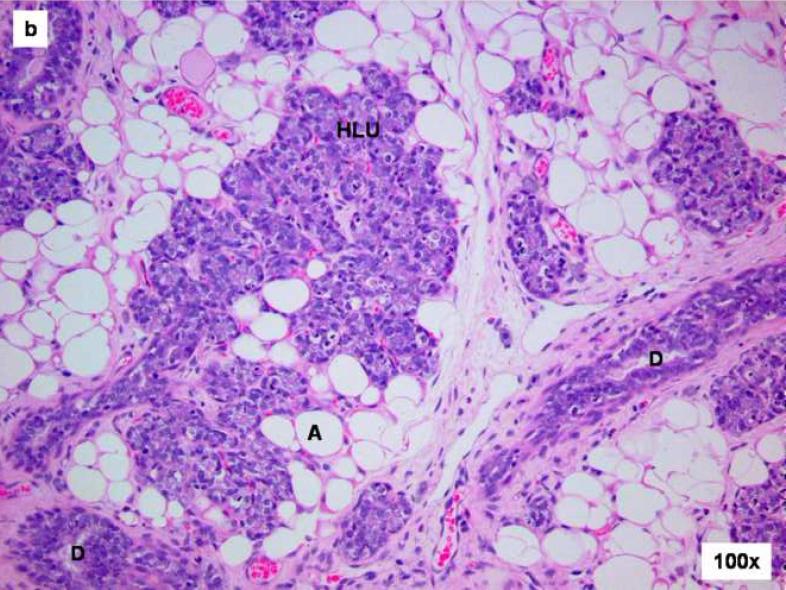
Figure 2. Cystic papillary carcinoma in E2-treated mammary gland after 120 days treatment.
Female ACI rats were treated with an E2 pellet (s.c., 3 mg E2 + 17 mg cholesterol) for 120 days. The ductal structures are markedly dilated (compare with uninvolved hyperplastic lobular unit (HLU) at the top of image). The neoplastic epithelium grows into the lumen forming papillae and rigid arches devoid of fibrovascular cores. The neoplastic cells are enlarged and generally uniform in size. In addition to the hypertrophic and hyperplastic changes, E2 treatment results in the appearance of malignant lesions. The mammary tissue of one animal showed cystic papillary carcinoma (CA) following 120 days of E2 treatment.
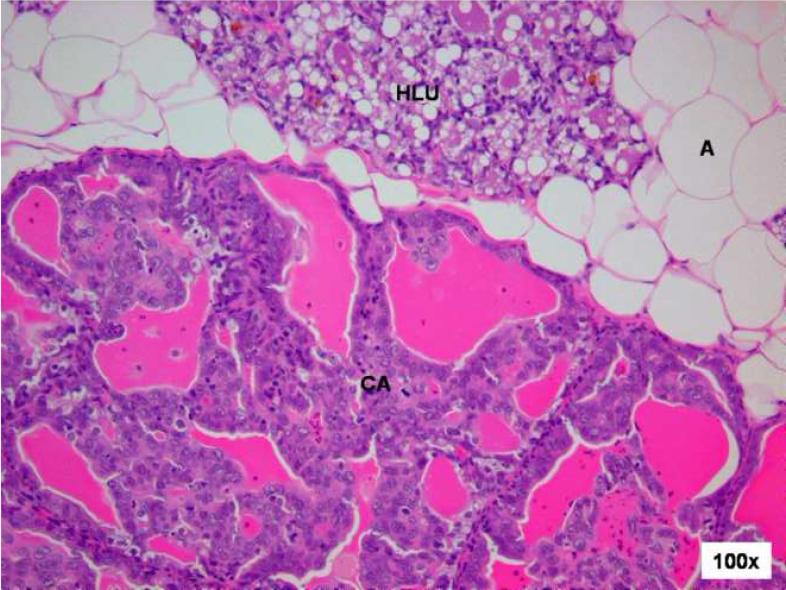
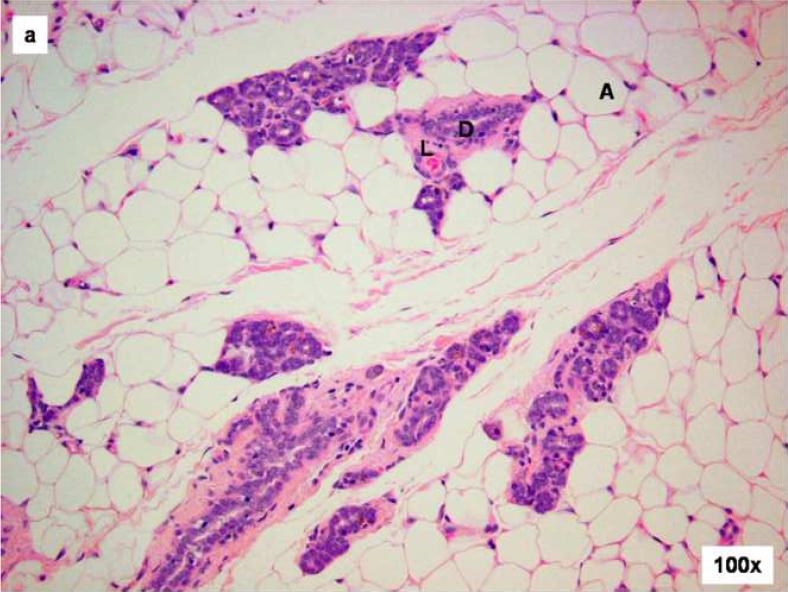
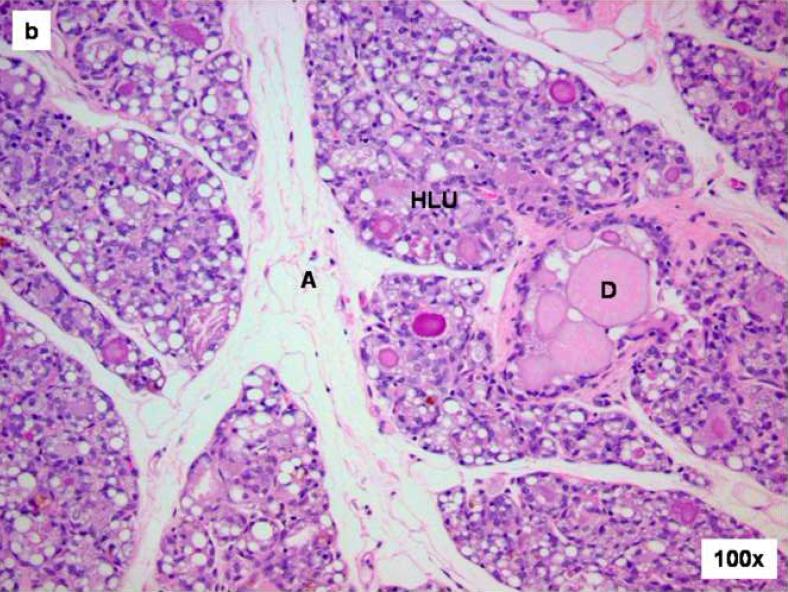
Figure 3. Comparison of control and E2-treated mammary gland after 240 days.
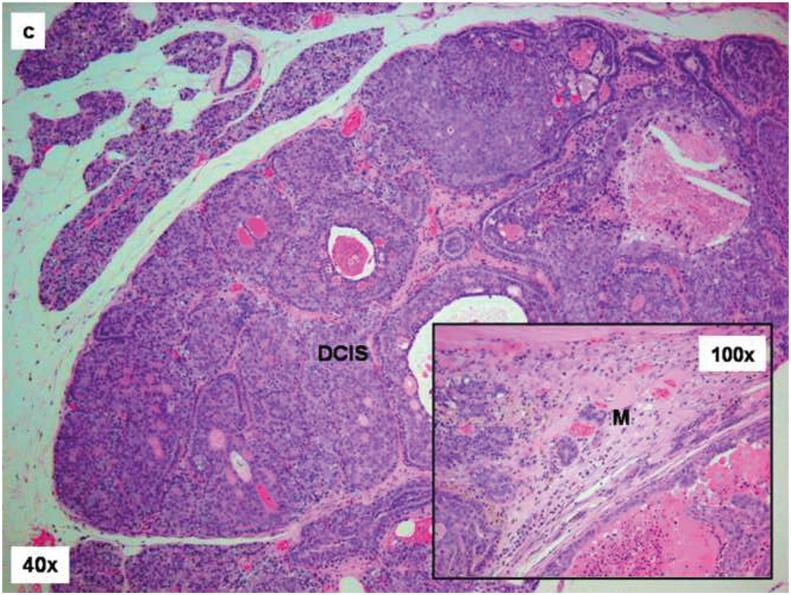
Female ACI rats were treated with an E2 pellet (s.c., 3 mg E2 + 17 mg cholesterol) for 240 days. Control rats were implanted with a pellet containing 17 mg cholesterol only. Histopathologic analyses were performed on mammary tissue from all control and E2-treated animals. This figure shows mammary tissue representative of the morphology generally observed in control and E2-treated animals. a) The mammary gland of control ACI rats shows normal lobular architecture (L) with branched ducts (D) and normal distribution of fat tissue (A). The control mammary tissue at 240 days is unchanged relative to earlier time-points. b) The E2-stimulated mammary gland shows increased proliferation with dilated ducts containing inspissated secretions (D) and increased proliferation and expansion of terminal lobular units (HLU) accompanied by compression of and expansion into the surrounding fat tissue (A). c) Histomorphologic analysis revealed carcinomas, both in situ (DCIS) and micro-invasive, measuring 10 or more mm in the largest dimension. The carcinomas showed a mixed morphology, with solid patterns with or without comedo-necrosis predominating, but cribriform and cystic-papillary patterns were also present. (40 × image) When present, the invasion was limited to foci of micro-invasion. 100x Inset) Microinvasive foci (M) were characterized by breakdown of the basal membrane of the in situ lesion by a proliferation of atypical small glands which invaded the surrounding stroma surrounded by a prominent desmoplastic reaction.
Female ACI rats exposed to E2 developed mammary tumors
The incidence of mammary tumors in female ACI rats treated with E2 reached 82% (9/11) following 240 days exposure. None of the animals in the control group developed tumors. The first palpable mammary tumor appeared in the E2 group after 128 days exposure. The number of tumor nodules in tumor-bearing animals ranged from 1 to 6, with an average of 2.9 ± 1.4 nodules per tumor-bearing rat. The average tumor size in E2-treated rats was 1086 ± 472 mm3.
8-isoPGF2α levels were elevated as a function of E2 exposure in female ACI rats
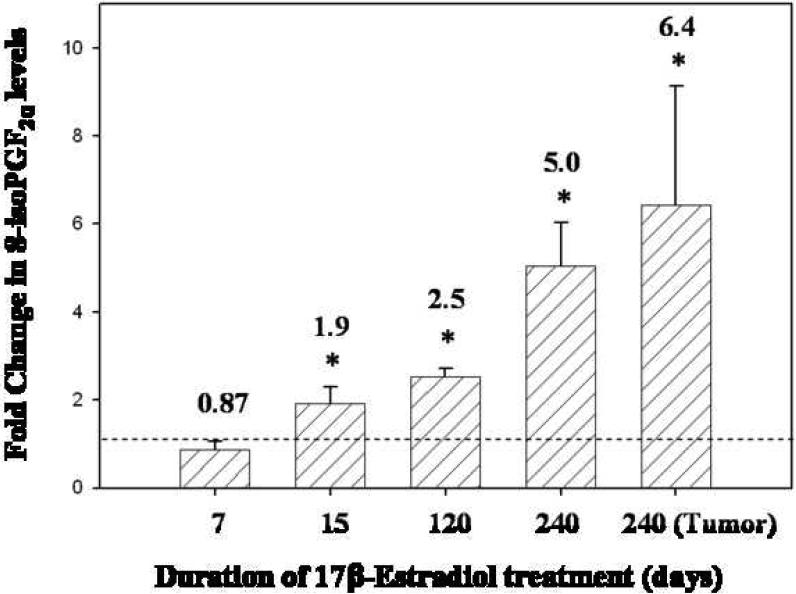
Following exposure to E2 for 15, 120 or 240 days, mammary 8-isoPGF2α levels were significantly increased over levels detected in age-matched control rats (Figure 4). 8-isoPGF2α levels were 1.9-fold higher in rats exposed to E2 for 15 days compared to age-matched control animals (p < 0.05). Similarly, 2.5 and 5-fold elevations in 8-iso-PGF2α levels were observed in rats treated with E2 for 120 and 240 days compared to age-matched controls, respectively (p < 0.05). In addition, 8-isoPGF2α levels were increased 6-fold in tissue from E2-induced mammary tumors relative to age-matched control mammary tissue (p < 0.05) (Figure 4). Increases in 8-isoPGF2α levels as a function of E2 exposure have not been previously reported in an animal model of estrogen-induced mammary cancer.
Figure 4. 8-isoPGF2α levels in ACI rat mammary tissue are increased in a time-dependent fashion after E2 exposure.
Female ACI rats were treated with E2 as described in the methods section. 8-isoPGF2α levels were measured in mammary tissue from control and E2-treated rats from the 7, 15, 120 and 240 day treatment groups. 8-isoPGF2α levels were assessed in mammary tumor tissue as well. Fold changes were calculated for E2-treated animals relative to age-matched controls. Fold change data are reported as an average of values obtained for at least 7 different animals ± SE. The absolute values of 8-isoPGF2α (pg 8-isoPGF2α per mg protein ± SE) in mammary tissue from control rats in the 7, 15, 120 and 240 day groups were 1590 ± 300, 511 ± 87, 1320 ± 370 and 492 ± 100, respectively. * indicates a p value <0.05 compared to respective age-matched controls.
Activities of antioxidant enzymes SOD and GPx were altered in response to E2 exposure
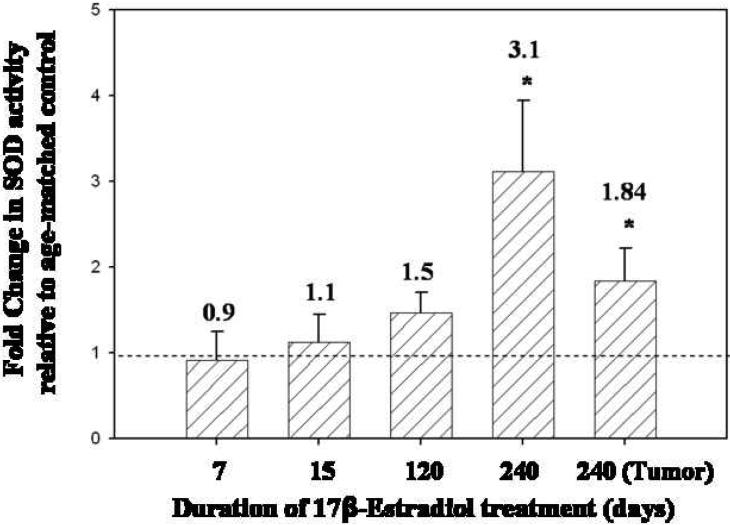
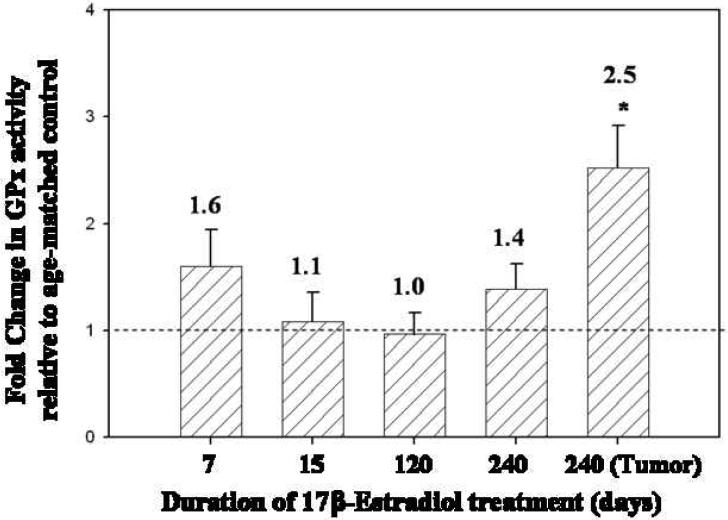
SOD activity levels in the mammary tissue of rats treated with E2 for 7, 15 and 120 days were not significantly different from SOD activity levels found in age-matched controls (Figure 5). However, mammary tissue from rats treated with E2 for 240 days, displayed a 3.1-fold increase in SOD activity relative to age-matched controls (p <0.05) (Figure 5). Moreover, SOD activity was 1.8 times higher in mammary tumor tissue than in age-matched control mammary tissue (p <0.05) (Figure 5). No differences in SOD activity levels were detected between E2-treated and control rat livers (Table 1). GPx activity levels in the mammary tissue of ACI rats treated with E2 for 7, 15, 120 and 240 days did not differ significantly from levels detected in age-matched control rats (Figure 6). In contrast, GPx activity in E2-induced mammary tumors was elevated 2.5-fold over levels detected in age-matched control rats (Figure 6). GPx activity levels in the liver tissue of E2-treated rats after 240 days was significantly higher than levels observed in control rats (Table 1). CAT activity levels in mammary tissue from rats treated with E2 for 7, 15, 120 or 240 days were not significantly different from levels observed in age-matched control rats (data not shown). In E2-induced mammary tumors, CAT activity was not significantly different from activity levels found in control mammary tissue.
Figure 5. SOD activity is elevated in the mammary tissue of E2-treated rats compared to age-matched controls.
Female ACI rats were treated with E2 as described in the methods section. The activity of the antioxidant enzyme SOD was measured in mammary tissue from control and E2-treated rats from 7, 15, 120 and 240 day treatment groups. SOD activity was assessed in mammary tumor tissue as well. Fold changes were calculated for E2-treated animals relative to age-matched controls.Fold change data are reported as an average of values obtained for at least 7 different animals ± SE. The absolute values of SOD activity (units per mg protein ± SE) in mammary tissue from control rats in the 7, 15, 120 and 240 day groups were 571 ± 220, 990 ± 240, 969 ± 150 and 561± 70, respectively. * indicates a p value <0.05 compared to respective age-matched controls.
Table 1.
Activities of antioxidant enzymes in 240 day old female ACI rats
| Tissue | SOD (U/mg protein) | CAT (nmol/min/mg protein) | GPx (nmol/min/mg protein) |
|---|---|---|---|
| Female ACI rats were treated with an E2 pellet (s.c., 3 mg E2 + 17 mg cholesterol) for 240 days. Control rats were implanted with a pellet containing 17 mg cholesterol only. The activities of the antioxidant enzymes SOD, GPx and CAT were measured in mammary and liver tissue from control and E2-treated rats from the 240 day treatment group. Enzyme activity was assessed in mammary tumor tissue as well. The data are reported as an average of values obtained for at least 7 different animals ± SE. | |||
| Control Mammary | 561 ± 70 | 88 ± 9 | 92 ± 9 |
| E2-Treated Mammary | 1747 ± 460* | 104 ± 12 | 128 ± 22 |
| Mammary Tumor | 1031 ± 211* | 65 ± 5 | 232 ± 37* |
| Control Liver | 169 ± 11 | 1290 ± 170 | 601 ± 88 |
| E2-Treated Liver | 152 ± 13 | 1270 ± 350 | 1170 ± 81* |
Indicates a p value <0.05 compared to control mammary or liver compared to respective age-matched controls.
Figure 6. GPx activity levels are increased in mammary tumors compared to adjacent normal mammary tissue.
Female ACI rats were treated with E2 as described in the methods section. The activity of the antioxidant enzyme GPx was measured in mammary tissue from control and E2-treated rats from 7, 15, 120 and 240 day treatment groups. GPx activity was assessed in mammary tumor tissue as well. Fold changes were calculated for E2-treated animals relative to age-matched controls. Fold change data are reported as an average of values obtained for at least 7 different animals ± SE. The absolute values of GPx activity (nmol per minute per mg protein ± SE) in mammary tissue from control rats in the 7, 15, 120 and 240 day groups were 49 ± 9, 65 ± 10, 94 ± 10 and 92 ± 9, respectively. * indicates a p value <0.05 compared to respective age-matched controls.
Discussion
In the present study, we used the ACI rat model of breast cancer to examine the biochemical and histopathologic changes that occur in mammary tissue following estrogen exposure. Specifically, we set out to assess the contribution of oxidative stress to the pathogenesis of estrogen-induced breast tumorigenesis. We examined both proliferative changes and oxidative stress markers as a function of length of exposure to E2 in order to discern the association between oxidative stress and exposure to estrogen. In addition, we quantified oxidative and proliferative changes during the pre-neoplastic and neoplastic phases of E2 exposure. While proliferative changes in mammary tissue following prolonged exposure to E2 have previously been reported, early changes, such as those that occur after 7 or 15 days of E2 exposure, have not yet been reported in the literature. The findings of the present study describe the progression from normal mammary tissue to mammary cancer in the ACI rat model. These data suggest a progression from normal mammary tissue to proliferative tissue, such as atypical ductal hyperplasia, later progressing to tumor formation and malignancy. The initial proliferative stage was observed following 7 to 15 days of E2 exposure. The proliferative process became atypical after 120 days of E2 exposure, and the first palpable tumor appeared after 128 days of E2 treatment.
Levels of 8-isoPGF2α and activities of antioxidant enzymes including SOD, CAT and GPx were analyzed in the mammary tissue of E2-treated and control ACI rats in order to evaluate oxidative stress. Oxidative stress is characterized by an imbalance between ROS and antioxidant defense systems that control the production of free radicals and ROS (Cracowski et al., 2002). Oxidative stress and subsequent oxidative damage to biomolecules, such as DNA, protein and lipids, has been identified as a salient feature of numerous acute and chronic diseases, including cancer (Cracowski et al., 2002; Bhat et al., 2003; Patel and Bhat, 2004; Milne et al., 2008). 8-isoPGF2α, an isoprostane produced from arachidonic acid during lipid peroxidation, was used as a marker of oxidative stress in ACI rat tissue. Quantification of 8-isoPGF2α is recognized as a reliable method of assessing oxidative stress and oxidative damage in vivo (Montuschi et al., 2004; Milne et al., 2007). Free radical-induced peroxidation of membrane lipids can disturb the physical properties of the lipid membrane, and thus interferes with normal cellular functioning (Montuschi et al., 2004; Milne et al., 2008). The observation that 8-isoPGF2α levels increased in a time-dependent manner following E2 exposure suggests that E2 induces oxidative stress, in particular lipid peroxidation, prior to tumor development. After just 15 days treatment with E2, 8-isoPGF2α levels were elevated nearly 2-fold over levels observed in age-matched controls, indicating that lipid peroxidation represents an early event following E2 exposure. By 240 days,8-isoPGF2α levels in E2-exposed mammary tissues were substantially increased, reaching levels 5-times higher than those observed in control mammary tissue (Figure 4). Moreover, E2-induced mammary tumors displayed significant elevations in 8-isoPGF2α levels relative to control mammary tissue. These changes are indicative of lipid peroxidation and possible cellular damage in the mammary gland as a result of E2 exposure. Elevations in 8-isoPGF2α appeared to be confined to E2-target tissues, as no changes in this marker were detected in the liver tissue of E2-treated rats relative to controls (data not shown).
Antioxidant enzymes represent an important defense mechanism against oxidative stress. SOD, GPx and CAT activities were assessed in E2-exposed and control ACI rats as a means of further assessing oxidative stress and the response to E2 exposure. SOD catalyzes the dismutation of superoxide to generate hydrogen peroxide, while CAT is responsible for the decomposition of hydrogen peroxide to oxygen and water. Similarly, GPx eliminates hydrogen peroxide and organic peroxides by way of reduced glutathione. The relationship between SOD and the process of carcinogenesis remains uncertain. Studies have reported that MnSOD protein levels increase with tumor grade and other investigations have correlated increased MnSOD activity with de-differentiation (Tsanou et al., 2004; Kumaraguruparan et al., 2005; Kattan et al., 2008). Similarly, Western blotting of human breast cancer tissues revealed that MnSOD expression was 1.5-fold higher in cancer tissue compared to adjacent normal tissue (Er et al., 2004). In vitro experiments have shown increased MnSOD activity in non-aggressive and aggressive breast cancer cells relative to non-tumorigenic mammary epithelial cells (Mukhopadhyay et al., 2004) In contrast, MnSOD over-expression has been associated with decreased cell growth and survival in vitro and with decreased tumor growth in xenograft models of breast cancer (Weydert et al., 2006). Using the ACI rat model of breast cancer, we have shown that SOD activity was significantly increased in mammary tissue from rats exposed to E2 for 240 days as well as in mammary tumor tissue (Figure 5). This finding implies a compensatory mechanism in response to oxidative stress that may represent a step in the process of tumorigenesis. Moreover, up-regulation of antioxidant enzymes may confer a selective advantage for the growth of tumor cells compared to corresponding normal cells.
Further supporting a role for oxidative stress in the pathogenesis of E2-induced mammary cancer, we detected a significant increase in GPx activity in the mammary tumor tissue of E2-exposed rats (Figure 6). While GPx activity in non-tumor mammary tissue was not significantly impacted by E2 exposure at any of the time points tested, our data suggest the possibility of an early increase in GPx activity after 7 days treatment, followed by a leveling off of GPx activity after prolonged E2 exposure (Figure 6). Increases in SOD activity without parallel increases in GPx and CAT activity may result in elevated hydrogen peroxide production without sufficient detoxifying enzymes, thus magnifying the burden of oxidative stress in the cell. Previous reports reveal higher GPx activity in human breast tumors compared to adjacent normal tissue, and microarray studies have shown that GPx2 is commonly over-expressed in mammary carcinomas. In vitro experiments have found that suppressing GPx2 is associated with growth inhibition in rat and human mammary cancer cell lines (di Ilio et al., 1985; Singh et al., 1990; Gromadzinska et al., 1997; Iscan et al., 1998; Naiki-Ito et al., 2007). However, the level of GPx in tumor tissue does not seem to be altered in all cases (Singh et al., 1990). The lack of elevation in GPx activity perhaps reflects that this anti-oxidant pathway is only triggered late in the process of carcinogenesis. Hence, at early time-points (i.e. during proliferation and atypical ductal proliferation) the GPx anti-oxidant mechanism is unaffected.
Taken together, our data indicate that oxidative stress plays an important role in the development of estrogen-induced mammary cancer. Increases in lipid peroxidation and up-regulation of antioxidant enzymes precede tumor development and occur in a manner dependent on duration of exposure to E2.
Acknowledgements
We would like to thank Dr. Xinhua Liu for her statistical expertise and help with this manuscript. This work was supported by the National Institutes of Health Grants ES009089 and CA 109551 (HKB). The authors have no conflict of interest at this time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnerlov C, Emdin SO, Cajander S, Bengtsson NO, Tavelin B, Roos G. Intratumoral variations in DNA ploidy and s-phase fraction in human breast cancer. Anal. Cell. Pathol. 2001;23:21–28. doi: 10.1155/2001/430674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50:1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemeier RW, Bhat AS, Lovely CJ, Coughenour HD, Joomprabutra S, Weitzel DH, Vandre DD, Yusuf F, Burak WE., Jr. 2-Methoxymethylestradiol: a new 2-methoxy estrogen analog that exhibits antiproliferative activity and alters tubulin dynamics. J. Steroid Biochem. Mol. Biol. 2001;78:145–156. doi: 10.1016/s0960-0760(01)00090-5. [DOI] [PubMed] [Google Scholar]
- Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine, and glutathione. Chem. Res. Toxicol. 1998;11:909–916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Cavalieri EL, Rogan EG. A unifying mechanism in the initiation of cancer and other diseases by catechol quinones. Ann. N.Y. Acad. Sci. 2004;1028:247–257. doi: 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons M, Goss P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol. Sci. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- di Ilio C, Sacchetta P, del Boccio G, la Rovere G, Federici G. Glutathione peroxidase, glutathione S-transferase and glutathione reductase activities in normal and neoplastic human breast tissue. Cancer Lett. 1985;29:37–42. doi: 10.1016/0304-3835(85)90120-x. [DOI] [PubMed] [Google Scholar]
- Er TK, Hou MF, Tsa EM, Lee JN, Tsai LY. Differential expression of manganese containing superoxide dismutase in patients with breast cancer in Taiwan. Ann. Clin. Lab. Sci. 2004;34:159–164. [PubMed] [Google Scholar]
- Felty Q, Singh KP, Roy D. Estrogen-induced G1/S transition of G0-arrested estrogen-dependent breast cancer cells is regulated by mitochondrial oxidant signaling. Oncogene. 2005a;24:4883–4893. doi: 10.1038/sj.onc.1208667. [DOI] [PubMed] [Google Scholar]
- Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005b;44:6900–6909. doi: 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics:significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Gromadzinska J, Wasowicz W, Andrijewski M, Sklodowska M, Quispe OZ, Wolkanin P, Olborski B, Pluzanska A. Glutathione and glutathione metabolizing enzymes in tissues and blood of breast cancer patients. Neoplasma. 1997;44:45–51. [PubMed] [Google Scholar]
- Han X, Liehr JG. DNA single-strand breaks in kidneys of Syrian hamsters treated with steroidal estrogens: hormone-induced free radical damage preceding renal malignancy. Carcinogenesis. 1994;15:997–1000. doi: 10.1093/carcin/15.5.997. [DOI] [PubMed] [Google Scholar]
- Harvell DM, Strecker TE, Tochacek M, Xie B, Pennington KL, McComb RD, Roy SK, Shull JD. Rat strain-specific actions of 17β-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2779–2784. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC . Cancer incidence in five continents. Volume 9. International Agency for Research on Cancer; Lyon: 2007. [Google Scholar]
- IARC . IARC monographs on the evaluation of carcinogenic risks to humans: overall evaluations of carcinogenicity, an updating of IARC monographs. volumes 1 to 42. International Agency for Research on Cancer; Lyon: 1987. Supplement 7. [PubMed] [Google Scholar]
- IARC . IARC monographs on the evaluation of carcinogenic risks to humans: hormonal contraception and postmenopausal hormone therapy. Volume 72. International Agency for Research on Cancer; Lyon: 1999. pp. 474–530. [Google Scholar]
- Iscan M, Coban T, Bulbul D, Eke BC, Aygormez S, Berberoglu U. Xenobiotic metabolizing and antioxidant enzymes in normal and neoplastic human breast tissue. Eur. J.Drug Metab. Pharmacokinet. 1998;23:497–500. doi: 10.1007/BF03190001. [DOI] [PubMed] [Google Scholar]
- Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006;27:1292–1299. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- Kattan Z, Minig V, Leroy P, Dauca M, Becuwe P. Role of manganese superoxide dismutase on growth and invasive properties of human estrogen-independent breast cancer cells. Breast Cancer Res. Treat. 2008;108:203–215. doi: 10.1007/s10549-007-9597-5. [DOI] [PubMed] [Google Scholar]
- Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Kumaraguruparan R, Kabalimoorthy J, Nagini S. Correlation of tissue lipid peroxidation and antioxidants with clinical stage and menopausal status in patients with adenocarcinoma of the breast. Clin. Biochem. 2005;38:154–158. doi: 10.1016/j.clinbiochem.2004.10.012. [DOI] [PubMed] [Google Scholar]
- LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62:3691–3697. [PubMed] [Google Scholar]
- Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed. Proc. 1987;46:1858–1863. [PubMed] [Google Scholar]
- Li JJ, Papa D, Davis MF, Weroha SJ, Aldaz CM, El-Bayoumy K, Ballenger J, Tawfik O, Li SA. Ploidy differences between hormone- and chemical carcinogeninduced rat mammary neoplasms: comparison to invasive human ductal breast cancer. Mol. Carcinog. 2002a;33:56–65. doi: 10.1002/mc.10022. [DOI] [PubMed] [Google Scholar]
- Li JJ, Weroha SJ, Lingle WL, Papa D, Salisbury JL, Li SA. Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc. Natl. Acad. Sci. U.S.A. 2004;101:18123–18128. doi: 10.1073/pnas.0408273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SA, Weroha SJ, Tawfik O, Li JJ. Prevention of solely estrogen-induced mammary tumors in female aci rats by tamoxifen: evidence for estrogen receptor mediation. J. Endocrinol. 2002b;175:297–305. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J. Steroid Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- Liehr JG, Han X, Bhat HK. 32P-postlabelling in studies of hormonal carcinogenesis. IARC Scientific Publications. 1993;124:149–155. [PubMed] [Google Scholar]
- Makris A, Allred DC, Powles TJ, Dowsett M, Fernando IN, Trott PA, Ashley SE, Ormerod MG, Titley JC, Osborne CK. Cytological evaluation of biological prognostic markers from primary breast carcinomas. Breast Cancer Res. Treat. 1997;44:65–74. doi: 10.1023/a:1005717924761. [DOI] [PubMed] [Google Scholar]
- McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321:624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J. Biol. Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Das SK, Mukherjee S. Expression of Mn-superoxide dismutase gene in nontumorigenic and tumorigenic human mammary epithelial cells. J Biomed. Biotechnol. 2004;2004:195–202. doi: 10.1155/S1110724304401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki-Ito A, Asamoto M, Hokaiwado N, Takahashi S, Yamashita H, Tsuda H, Ogawa K, Shirai T. Gpx2 is an overexpressed gene in rat breast cancers induced by three different chemical carcinogens. Cancer Res. 2007;67:11353–11358. doi: 10.1158/0008-5472.CAN-07-2226. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- NTP . Report on Carcinogens. National Toxicology Program; Research Triangle Park: 2002. [Google Scholar]
- Patel MM, Bhat HK. Differential oxidant potential of carcinogenic and weakly carcinogenic estrogens: Involvement of metabolic activation and cytochrome P450. J. Biochem. Mol. Toxicol. 2004;18:37–42. doi: 10.1002/jbt.20005. [DOI] [PubMed] [Google Scholar]
- Ries L, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK. SEER Cancer Statistics Review. National Cancer Institute; Bethesda: 2007. pp. 1975–2004. [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Roy D, Cai Q, Felty Q, Narayan S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J. Toxicol. Environ. Health B. Crit. Rev. 2007;10:235–257. doi: 10.1080/15287390600974924. [DOI] [PubMed] [Google Scholar]
- Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovaryintact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- Singh SV, Brunnert SR, Roberts B, Krishan A. Differential expression of glutathione S-transferase, glutathione peroxidase and glutathione reductase in normal and malignant human breast tissues. Cancer Lett. 1990;51:43–48. doi: 10.1016/0304-3835(90)90229-q. [DOI] [PubMed] [Google Scholar]
- Tsanou E, Ioachim E, Briasoulis E, Damala K, Charchanti A, Karavasilis V, Pavlidis N, Agnantis NJ. Immunohistochemical expression of superoxide dismutase (MnSOD) anti-oxidant enzyme in invasive breast carcinoma. Histol. Histopathol. 2004;19:807–813. doi: 10.14670/HH-19.807. [DOI] [PubMed] [Google Scholar]
- Wang MY, Liehr JG. Induction by estrogens of lipid peroxidation and lipid peroxide-derived malonaldehyde-DNA adducts in male Syrian hamsters: role of lipid peroxidation in estrogen-induced kidney carcinogenesis. Carcinogenesis. 1995;16:1941–1945. doi: 10.1093/carcin/16.8.1941. [DOI] [PubMed] [Google Scholar]
- Weroha SJ, Li SA, Tawfik O, Li JJ. Overexpression of cyclins D1 and D3 during estrogen-induced breast oncogenesis in female ACI rats. Carcinogenesis. 2006;27:491–498. doi: 10.1093/carcin/bgi278. [DOI] [PubMed] [Google Scholar]
- Weydert CJ, Waugh TA, Ritchie JM, Iyer KS, Smith JL, Li L, Spitz DR, Oberley LW. Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic. Biol. Med. 2006;41:226–237. doi: 10.1016/j.freeradbiomed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J. Natl.Cancer Inst. Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Ziegler RG, Rossi SC, Fears TR, Bradlow HL, Adlercreutz H, Sepkovic D, Kiuru P, Wahala K, Vaught JB, Donaldson JL, Falk RT, Fillmore CM, Siiteri PK, Hoover RN, Gail MH. Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. Environ. Health Perspect. 1997;105(Suppl 3):607–614. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]