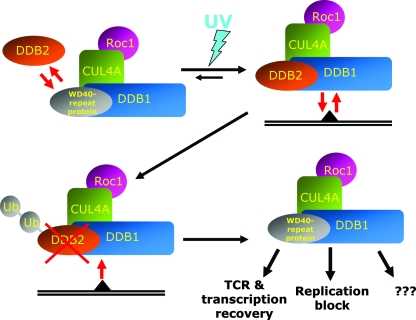
FIG. 6.
Hypothesis proposing the role of UV-induced DDB2 degradation in release of DDB1 for the interactions outside NER. Without UV irradiation, different WD40 repeat proteins associate with DDB1-ubiquitine ligase complexes. In the presence of the unrepaired DNA lesion, equilibrium is shifted toward assembly of the DDB2-DDB1-CUL4A-ROC1 complex, which constantly rebinds to the damaged DNA. At the same time, binding of the DDB2-containing ubiquitin ligase complex to UV-damaged DNA triggers ubiquitylation and proteasomic degradation of DDB2, which makes DDB1 available for inclusion in other E3 complexes, providing cellular response to the genotoxic stress.