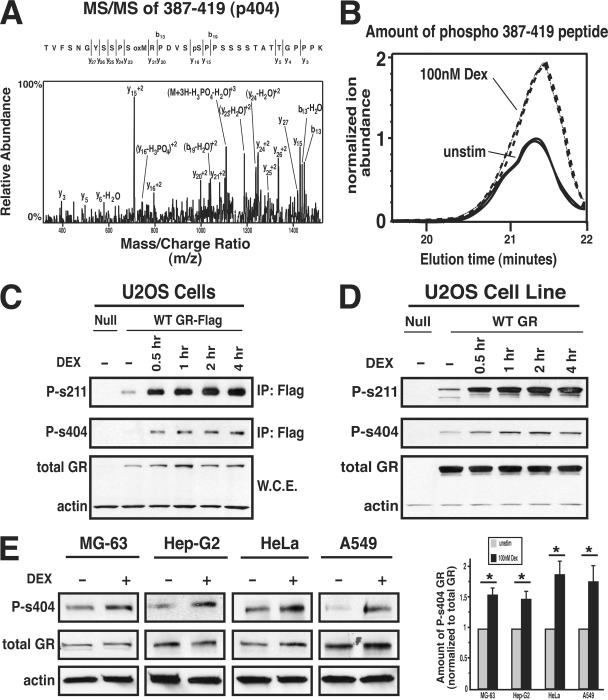
FIG. 1.
The GR is phosphorylated on Ser404. (A) Samples from control or Dex (100 nM; 1 h)-treated U-2 OS cells expressing Flag-tagged human GRα were resolved by gel electrophoresis, and the band containing GR was isolated and analyzed by mass spectrometry. The tandem mass spectrometry spectrum from the peptide containing GR phosphorylated on Ser404 is shown. (B) The ion abundance of phospho-387-419AA peptide in unstimulated and Dex-treated samples. (C) U-2 OS cells transiently expressing hGRα-Flag were treated with 100 nM Dex for 0 to 4 h, immunoprecipitated, and probed with phospho-specific and total anti-GR antibodies. (D) Null U-2 OS cells or U-2 OS cells stably expressing hGRα were treated with 100 nM Dex, and cell lysates were then probed with phospho-specific and total anti-GR antibodies. (E) Cells of the bone (MG-63), liver (Hep-G2), cervix (HeLa), and lung (A549) were treated with 100 nM Dex for 1 h, and lysates were analyzed by Western blotting to determine whether endogenous GR was phosphorylated at Ser404. The levels of P-s404 (GR phosphorylated on serine 404) from three independent experiments were quantified and normalized to total GR levels (*, P < 0.05).