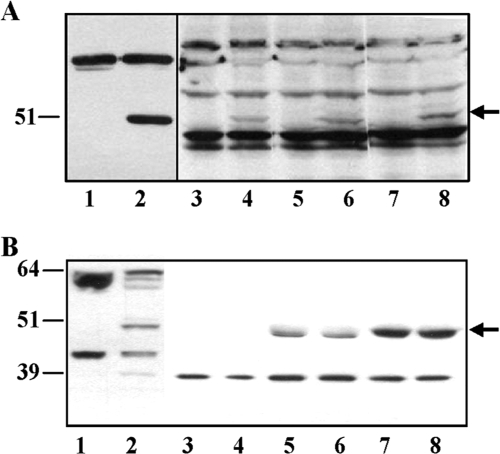
FIG. 6.
Expression of GTPBP3 in HEK-293 cells. (A) Immunoblotting using anti-GTPBP3 of extracts from HEK-293 cells nontransfected (lanes 3, 5, and 7) or permanently transfected with plasmid pIC1055 (lanes 4, 6, and 8) or from E. coli strain DH5α/pIC1296 grown in the absence (lane 1) or in the presence (lane 2) of inducer (arabinose). Samples were separated by 10% SDS-PAGE, and the amounts of bulk protein which were analyzed in each lane were as follows: 100 μg (lanes 1 and 2), 200 μg (lanes 3 and 4), 300 μg (lanes 5 and 6), and 400 μg (lanes 7 and 8). Signals were visualized by ECL (lanes 1 and 2) and ECL Advance (lanes 3 and 8) from GE Healthcare, and during film exposure each pair of lanes (1 and 2, 3 and 4, 5 and 6, and 7 and 8) was independently handled until optimal visualization of GTPBP3 bands was achieved. Nonspecific bands show equal loading in each pair of lanes (paired as above). Position and size (in kDa) of FLAG-(ΔN)GTPBP3 (detected in lane 2) are indicated on the left, and the arrow marks GTPBP3(Ins8A) position (detected in lanes 4, 6, and 8). (B) Extracts from HEK-293 cells permanently transfected or not with pIC1055 (approximately 2 or 5 mg of protein, respectively) were immunoprecipitated using anti-GTPBP3 as described in Materials and Methods. Immunoprecipitates and total extracts were resolved in a NuPAGE 4 to 12% Bis-Tris gel (Invitrogen) and immunoblotted with anti-GTPBP3 and rabbit True-blot (eBioscience, Inc.). Lane 1, total extract of nontransfected HEK-293 cells (100 μg); lane 2, total extract of HEK-293 cells permanently transfected with plasmid pIC1055 (50 μg); lanes 3 and 4, material bound to anti-rabbit IgG agarose beads (no antibody); lanes 5 and 6, immunoprecipitates of nontransfected HEK-293 cells; lanes 7 and 8, immunoprecipitates of HEK-293 cells transfected with plasmid pIC1055. Positions and sizes (in kDa) of mass markers are indicated on the left, and the arrow (on the right) marks GTPBP3 position.