Fig. 3.
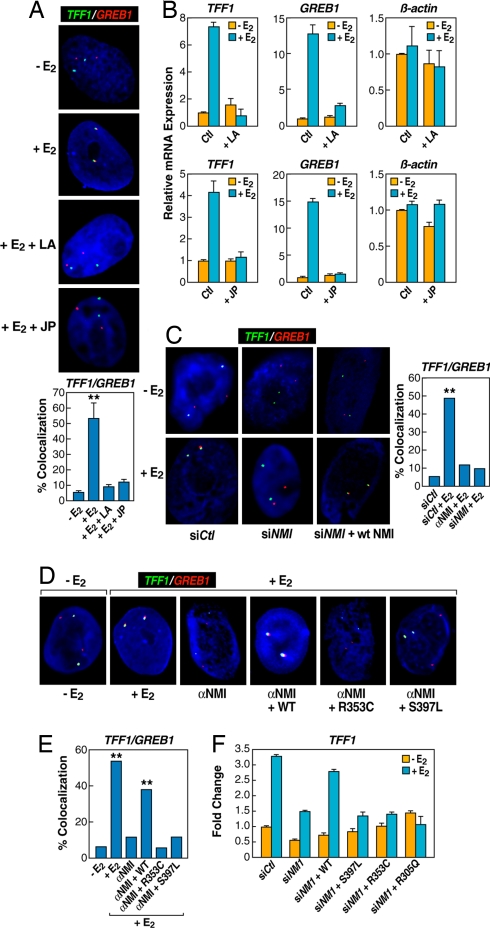
The nuclear actin/myosin machinery is required for long-distance chromosomal interactions. (A) Chemical disruption of actin polymerization with latrunculin (LA) (10 μM, 2 h), and prevention of actin depolymerization by jasplakinolide (JP) (10 μM, 2 h) impaired E2-induced interchromosomal interactions. The bar graph shows the percentage of cells ± SEM that showed colocalization under individual conditions (**, P < 0.001 by t test). (B) qPCR analysis of gene expression affected by JP and LA treatment. (C) Nuclear microinjection of siRNA or antibody against nuclear myosin I (NMI) abolished E2-induced TFF1:GREB1 interchromosomal interactions (**, P < 0.001 by χ2). (D and E) The requirement for the actin binding and ATPase activity of nuclear myosin I in mediating E2-induced interchromosomal interactions. Cells injected with antibody against NMI were coinjected with the plasmid expressing either WT or mutant NMI containing specific mutations in the myosin “head,” which were shown to be critical for actin binding (R353C) and ATPase activity (S497L) of the motor protein (**, P < 0.001 by χ2). (F) Rescue of E2-induced expression of TFF1 by expression of WT, but not mutant, NMI. Results are the average of triplicates ± SD differing by <10%; similar results were observed in duplicate experiments.