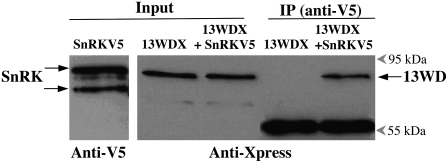
Figure 3.
13WD40-Xpress protein interacts with SnRK1.1-V5 in vitro. Immunoprecipitation (IP) was carried out using an anti-V5 antibody bound to a protein A-Sepharose (PAS). Purified and dialyzed proteins (13WDX and SnRKV5) were incubated for 2 h at room temperature in immunoprecipitation buffer before transferring to the antiV5:PAS complex. The resulting immunoprecipitation fractions were analyzed using protein gel blotting. Lane 1, Input fraction of SnRKV5 probed with an anti-V5 antibody; lane 2, input fraction of 13WDX; lane 3, input fraction of 13WDX in combination with SnRKV5; lane 4, bound fraction of 13WDX alone; lane 5, bound fraction of 13WDX in combination with SnRKV5. Lanes 2 through 5 were probed with an anti-Xpress antibody. The 55-kD band is the immunoglobin heavy chain component of the anti-V5 antibody. The blot images are representative of four independent experiments.