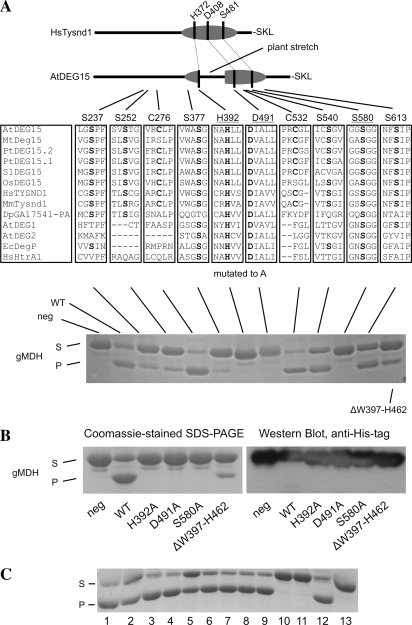
Figure 3.
Site-directed mutants demonstrate that the His-Asp-Ser catalytic triad carries out the proteolytic activity of Arabidopsis DEG15. A (top), Diagram of Homo sapiens TYSND1 with the location of the His (H372), Asp (D408), and Ser (S481) of the expected catalytic triad and the C-terminal Ser (S), Lys (K), and Leu (L) for the PTS1 signal. It is compared to Arabidopsis DEG15 with its plant-specific stretch between the catalytic His and Asp. Middle, Residues conserved in the majority of DEG15 homologs from Arabidopsis thaliana (At), Medicago truncatula (Mt), Populus tremula (Pt), Solanum lycopersicum (Sl), Oryza sativa (Os), H. sapiens (Hs), Mus musculus (Mm), Drosophila pseudoobscura (Dp), and the respective regions in Arabidopsis DEG1 and DEG2, Escherichia coli (Ec) DegP, and H. sapiens HtrA2. Residues are labeled according to their position in DEG15 from Arabidopsis. Residues forming the potential catalytic triad are underlined and residues mutated to Ala are bold. Bottom, Proteolytic activity of recombinant wild-type and mutated DEG15 toward purified His-6/XpressEpitope-tagged pre-gMDH. S, Substrate; P, processed gMDH. Additionally, a mutant with a deletion of the plant-specific loop (ΔW397-H462) was tested. Only mutation of H392, D491, and S580 to Ala completely eliminated processing. B, N-terminal processing of recombinant His6/XpressEpitope-tagged gMDH by DEG15. Left, Coomassie-stained SDS-PAGE gel. Right, Detection of the N-terminal His-tag by immunoblotting. The tag is absent in the processed substrate. C, Proteolytic activity of DEG15WT against recombinant pre-gMDH in the presence of protease inhibitors. S, Substrate; P, product of proteolysis. Shown are: positive control without inhibitors (line 1), 1 μm pepstatin A (line 2), 2 mm EGTA (line 3), 2 mm EDTA (line 4), 1 mm 1,10-phenanthroline (line 5), 1 mm benzamidine (line 6), 4 μg mL−1 aprotinin (line 7), 10 μm leupeptin (line 8), 20 μm E64 (line 9), 1 mm NEM (line 10), 2 mm NEM (line 11), 1 mm Pefabloc SC (line 12), and control reaction without protease (line 13).