Abstract
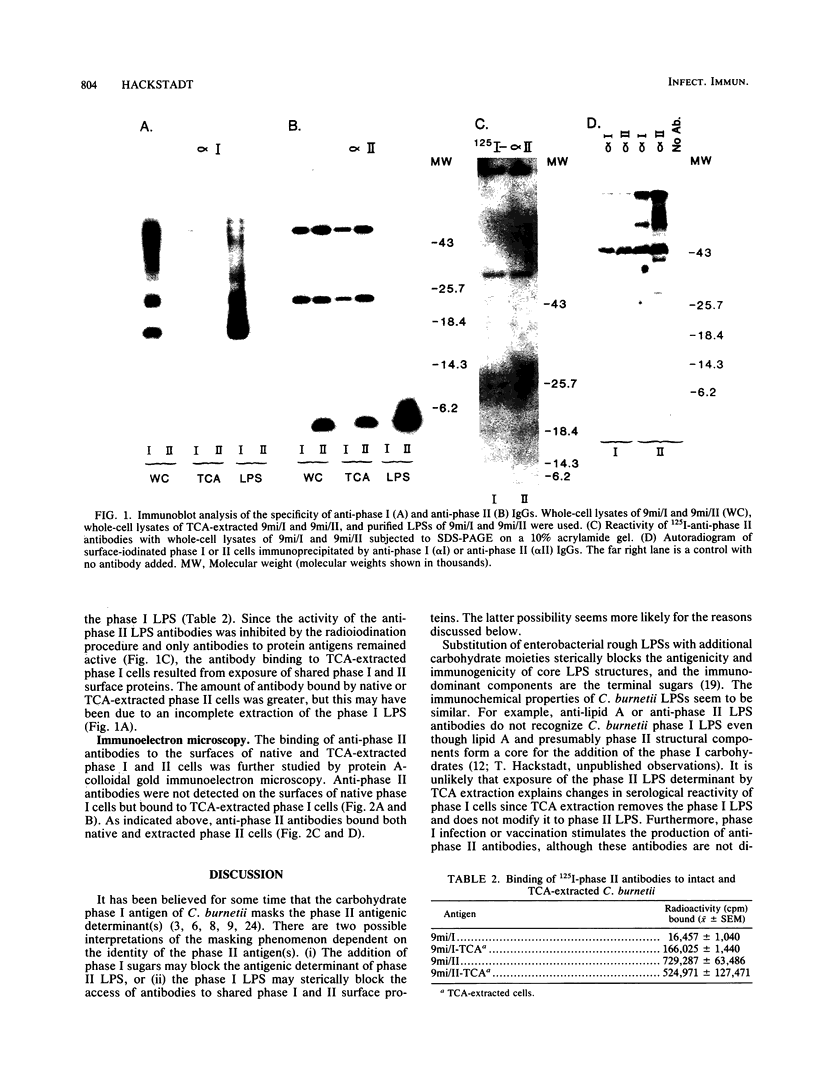
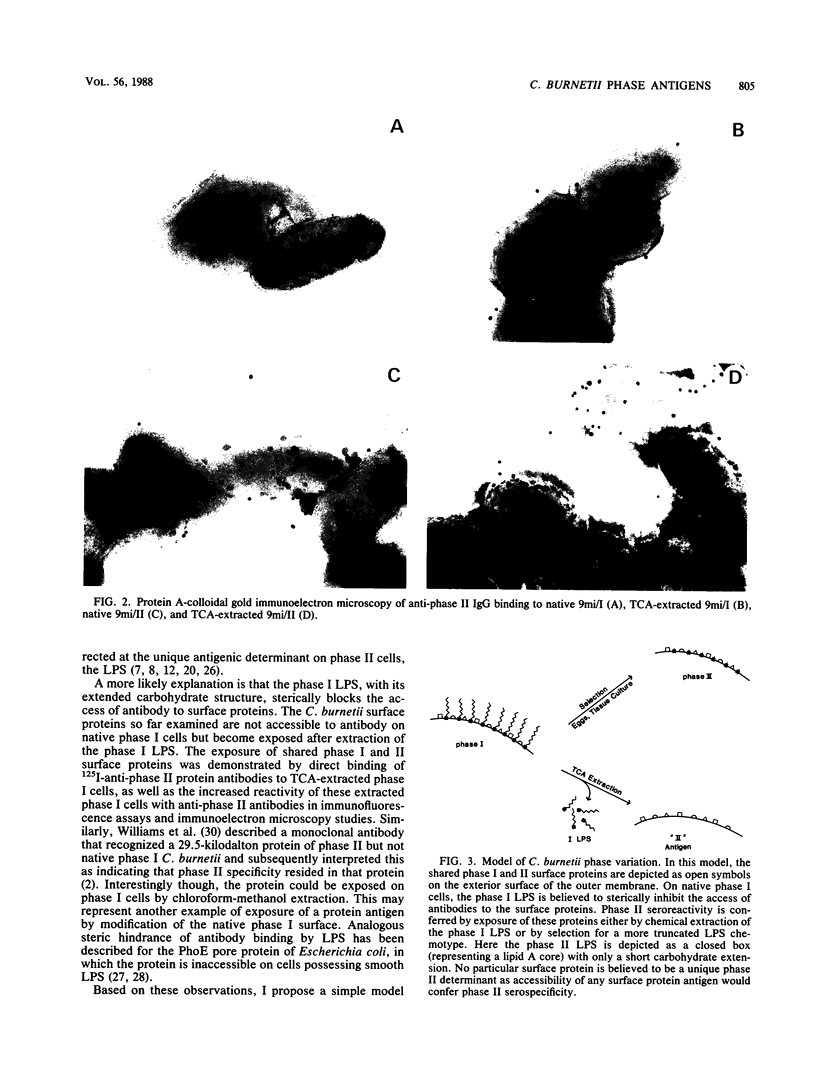
The exposure of surface protein antigens on virulent phase I Coxiella burnetti was compared with that on avirulent phase II cells. Although anti-phase II antibodies did not bind to the surfaces of native intact phase I cells, they bound to phase I proteins if the proteins were solubilized for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by immunoblotting. In addition, removal of the phase I lipopolysaccharide (LPS) by trichloroacetic acid exposed surface proteins for reactivity with anti-phase II antibodies, as shown by immunofluorescence assays, direct antibody binding, and immunoelectron microscopy using protein A-colloidal gold conjugates. Based on these observations, a simple model of phase variation is proposed to explain the apparently conflicting notions of the identity of the phase II antigen(s). The model suggests that the phase I LPS sterically hinders access of anti-phase II antibodies to a multitude of shared protein antigens, any one of which may confer phase II specificity. Exposure of these shared protein antigens through the appearance of a more truncated LPS (phase II) or extraction of the smooth-type phase I LPS allows antibody accessibility and therefore confers apparent phase II serospecificity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Williams J. C. Chemical and immunological characterization of lipopolysaccharides from phase I and phase II Coxiella burnetii. J Bacteriol. 1984 Dec;160(3):994–1002. doi: 10.1128/jb.160.3.994-1002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Williams J. C., McCaul T. F., Peacock M. G. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J Bacteriol. 1984 Dec;160(3):982–988. doi: 10.1128/jb.160.3.982-988.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca O. G., Paretsky D. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev. 1983 Jun;47(2):127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISET P. Phase variation of Rickettsia (Coxiella) burneti; study of the antibody response in guinea pigs and rabbits. Can J Microbiol. 1957 Apr;3(3):435–445. doi: 10.1139/m57-046. [DOI] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A. The antibody response to antigens of Coxiella burneti. Zentralbl Bakteriol Orig. 1968 Apr;206(3):321–329. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hackstadt T. Antigenic variation in the phase I lipopolysaccharide of Coxiella burnetii isolates. Infect Immun. 1986 Apr;52(1):337–340. doi: 10.1128/iai.52.1.337-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Peacock M. G., Hitchcock P. J., Cole R. L. Lipopolysaccharide variation in Coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985 May;48(2):359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Todd W. J., Caldwell H. D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J Bacteriol. 1985 Jan;161(1):25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs D. J., Jerrells T. R. In vitro evaluation of immunity to Coxiella burnetii. J Immunol. 1976 Sep;117(3):996–1003. [PubMed] [Google Scholar]
- Humphres R. C., Hinrichs D. J. Role of antibody in Coxiella burnetii infection. Infect Immun. 1981 Feb;31(2):641–645. doi: 10.1128/iai.31.2.641-645.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Burger G. T. Appearance of cellular and humoral immunity in guinea pigs after infection with Coxiella burnetii administered in small-particle aerosols. Infect Immun. 1977 May;16(2):518–521. doi: 10.1128/iai.16.2.518-521.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos A., Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun. 1987 May;55(5):1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B., TALLENT G. THE INFLUENCE OF PHASE ON THE PROTECTIVE POTENCY OF Q FEVER VACCINE. J Immunol. 1964 Mar;92:404–412. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Ormsbee R. A., Peacock M. G., Burgdorfer W. Microimmunofluorescence test for the serological study of rocky mountain spotted fever and typhus. J Clin Microbiol. 1976 Jan;3(1):51–61. doi: 10.1128/jcm.3.1.51-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- Schramek S., Brezina R., Kazár J. Influence of mild acid hydrolysis on the antigenic properties of phase I Coxiella burnetii. Acta Virol. 1978 Jul;22(4):302–308. [PubMed] [Google Scholar]
- Schramek S., Mayer H. Different sugar compositions of lipopolysaccharides isolated from phase I and pure phase II cells of Coxiella burnetii. Infect Immun. 1982 Oct;38(1):53–57. doi: 10.1128/iai.38.1.53-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhout W. F., Leunissen-Bijvelt J. J., Leunissen J. L., Verkleij A. J. Steric hindrance in immunolabelling. J Microsc. 1986 Mar;141(Pt 3):303–310. doi: 10.1111/j.1365-2818.1986.tb02724.x. [DOI] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Johnston M. R., Peacock M. G., Thomas L. A., Stewart S., Portis J. L. Monoclonal antibodies distinguish phase variants of Coxiella burnetii. Infect Immun. 1984 Jan;43(1):421–428. doi: 10.1128/iai.43.1.421-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Thomas L. A., Peacock M. G. Identification of phase-specific antigenic fractions of Coxiella burnetti by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Dec;24(6):929–934. doi: 10.1128/jcm.24.6.929-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- van der Ley P., Kuipers O., Tommassen J., Lugtenberg B. O-antigenic chains of lipopolysaccharide prevent binding of antibody molecules to an outer membrane pore protein in Enterobacteriaceae. Microb Pathog. 1986 Feb;1(1):43–49. doi: 10.1016/0882-4010(86)90030-6. [DOI] [PubMed] [Google Scholar]