Abstract
Drugs-of-abuse produce both acute and chronic changes in brain function, each of which is reflected in altered gene expression patterns. A number of large-scale gene expression studies have employed microarray analysis of human postmortem brain to identify transcriptional correlates of ante-mortem substance use. These studies have identified changes in transcripts encoding proteins functionally involved in neuronal function and synaptic plasticity, oligodendrocyte function and myelination, lipid and energy metabolism, mitochondrial function, oxidative phoshorylation, and cytoskeleton-related signal transduction. Overall, different types of substance use appear to share some of these effects, but there are more differences than similarities in gene expression for different types of substance use. Moreover, data suggest that transcriptional subtypes within a diagnostic classification of substance use may occur. These transcriptional subtypes, or “endophenotypes”, may reflect complex patterns of substance use and comorbid neuropsychiatric disorders or other disease, which may interact with substance use to differentially impact gene expression.
A broader understanding of the manner in which substance abuse causes long-term changes in brain function may be obtained from studies replicating and expanding the present gene expression data. In particular, cross-referencing comprehensive transcriptional data on regional and/or substance use-specific changes with genetic and proteomic data may further aid in identifying candidate biomarkers of altered brain function in substance use disorders.
Keywords: alcohol, cocaine, cannabis, gene expression, human postmortem brain, nicotine, RNA, substance abuse
Introduction
Substance use disorders are neuropsychiatric disorders1 characterized by increasing use and dependence on either licit (nicotine and alcohol) or illicit substances, such as cannabis, cocaine, heroin and phencyclidine, irrespective of adverse consequences. These substances produce both acute and long-lasting changes in the function of multiple brain regions2, which to some extent are reflected in altered transcriptional patterns. Characterizing transcriptional correlates of substance use may therefore provide an important avenue to gain information on the neurobiology underlying human substance use disorders.
Specific considerations in studies employing human postmortem brain
Examination of human postmortem brain currently provides the only direct manner in which the cellular neurobiology of ante-mortem substance use can be studied. Several biological variables and data analysis criteria influence optimal case selection and the ability to derive valid biological information from gene expression studies.
Case characterization
The effectiveness of applying inclusion and exclusion criteria to select index and control cases hinges in large part on the reliability of individual case histories. There are particular diagnostic challenges associated with reconstructing ante-mortem case history for deceased individuals.3–5 For these cases, case history, including characterization and diagnosis of the nature and extent of substance use, abuse or dependence on therapeutic and abused substances, relies on information from the Medical Examiner, availability of clinical/diagnostic information, and on second-hand accounts from next-of-kin. We found that a more detailed description of both lifetime and recent ante-mortem substance use was obtained when the Medical Examiner’s case history and toxicology was supplemented by comprehensive next-of-kin interviews and toxicological examination of several biological matrices, in particular scalp hair testing.5
Confounding factors
For any gene expression study, the quality of the starting material significantly impacts the ability to detect biologically relevant changes in gene expression. Multiple studies have established that high-quality RNA can be obtained from human postmortem brain6–10, even in cases with long postmortem intervals or with low brain pH. Ensuring a consistent and high level of RNA integrity has therefore become a standard quality control step prior to transcriptional analysis. Other potential confounding factors, such as agonal state/brain pH, age, gender, ethnicity, and smoking history, may differentially influence ante-mortem gene expression of individual transcripts and of specific groups of transcripts, and have to be addressed for each case-control comparison. Consideration of agonal state/brain pH is particularly important to ensure that reliable gene expression data can be extracted. The duration and extent of the agonal state appears to be inversely related to brain pH11, and a lower brain pH, indicating a protracted agonal state, has been associated with significant changes in transcriptional profile12, in particular of apoptotic, reactive oxygen stress, mitochondrial, chaperone and proteasome pathways.13 Furthermore, variations in respiratory stress in the acute agonal state during heroin overdose deaths differentially changed brain pH levels, and significantly impacted levels of proenkephalin and dopamine-related genes.14 Brain tissue from smokers appeared to have significantly lower brain pH than non-smokers9, which may skew expression data for transcripts such as mitochondrial genes that appear sensitive to agonal/pH state.13 Other potentially important sets of transcriptional differences are encoded by gender15–16, while the effects of age, ethnicity and postmortem interval are less clear-cut, but should all be addressed in the study design. Identifying potential confounds, and matching control and substance use cases accordingly, helps ensure that the transcriptional differences identified are not artifacts but representative of ante-mortem substance use.
Significance and relevance of gene expression data
Due to the cellular complexity of brain tissue, gene expression analysis often result in only modest fold-changes in expression, which further complicates establishing criteria for what constitutes a significant change. Significance has been defined in a number of different ways, such as arbitrarily preset cut-offs using fold-change17–18 or statistical significance19–24 criteria or a combination thereof.25–26 Most microarray studies of substance use employing human postmortem brain report hierarchical lists of “significantly changed” transcripts for a majority of individual case-control studies19–23 or for pooled groups of index and of control cases.17–18,24–25,27 The study design and manner of comparative analysis dictates what constitutes relevant change for a specific microarray platform. Although the use of different platforms, chemistries and data processing hampers direct comparisons between studies28, it is interesting to note that some individual transcripts and functional groups are repeatedly encountered across studies, substances, brain region and/or experimental cohorts (Table 1).
Table 1.
Transcripts reported to be changed in two or more microarray studies of postmortem brain from substance users. Nine gene expression microarray studies employing human postmortem brain from substance use cases17–25 were re-annotated using NCBI EntrezGene (http://www.ncbi.nlm.nih.gov/sites/gquery) using gene accession or Unigene identifiers. Transcripts, for which altered expression was reported in two or more different studies, are listed above by the official gene symbol. ▲ increased expression, ▼ decreased expression in substance use cases. Abbreviations: aPFC – anterior prefrontal cortex, dlPFC – dorsolateral prefrontal cortex, FC – frontal cortex, HIPP – hippocampus, supFC – superior frontal cortex.
| REGION | HIPP | supFC | supFC | dlPFC | NAc | FC | MCTX | NAc | NAc | dlPFC | aPFC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SUBSTANCE | NIC | EtOH | EtOH | EtOH | EtOH | EtOH | EtOH | HER | COC | COC | COC/THC/PCP |
| REFERENCE | 23 | 17 | 24 | 25 | 25 | 18 | 18 | 20 | 19 | 21 | 22 |
| MAG | - | ▽ | - | - | - | ▲▽ | ▲▽ | - | - | - | - |
| MBP | - | ▽ | - | - | - | - | - | - | ▽ | - | - |
| PLP1 | - | ▽ | ▽ | - | - | ▲▽ | ▲ | - | ▽ | ▲▽ | - |
| MOBP | - | - | - | - | - | ▲ | ▲ | - | ▽ | - | - |
| PMP22 | - | ▲▽ | ▽ | ▽ | ▽ | - | - | - | - | - | - |
| GPM6B | - | ▽ | - | - | - | ▲▽ | ▲ | - | - | ▲▽ | - |
| APOD | - | ▽ | - | ▽ | - | ▲▽ | ▲▽ | - | - | - | - |
| CLU | - | ▽ | - | ▲ | ▲ | - | - | - | - | - | - |
| CNP | - | ▲▽ | ▽ | - | - | - | - | - | - | - | - |
| UGT8 | - | ▽ | ▽ | - | - | - | - | - | - | - | - |
| ENPP2 | - | ▽ | - | - | - | ▲▽ | ▲▽ | - | - | - | - |
| CLTB | - | - | - | - | - | - | - | - | ▽ | ▲▽ | - |
| RTN1 | - | ▲ | - | - | - | ▲▽ | ▲ | - | - | - | - |
| LAMP2 | - | - | ▽ | - | ▽ | - | - | - | - | - | - |
| FOSB | - | - | - | - | - | ▲▽ | ▽ | - | - | - | - |
| SOX9 | - | ▽ | - | - | - | ▽ | ▲▽ | - | - | - | - |
| RPS10 | - | - | - | - | - | - | - | - | ▽ | - | ▲ |
| GFAP | - | ▽ | - | - | - | ▽ | ▲▽ | - | - | - | - |
| SDC2 | - | - | - | ▲ | - | ▽ | ▲▽ | - | - | - | - |
| CALM1 | ▲ | - | - | - | - | - | - | - | - | - | ▽ |
| CAPN3 | - | ▽ | ▽ | - | - | - | - | - | - | - | - |
| PPP3CB | ▲ | ▲ | - | - | - | ▲▽ | ▽ | - | - | - | - |
| NTRK2 | - | - | - | - | - | ▽ | ▲▽ | ▲ | ▽ | - | - |
| GNAO1 | ▲ | - | - | - | - | - | - | - | - | ▲▽ | - |
| GABBR1 | - | - | ▲ | ▲ | - | - | - | - | - | - | - |
| GRIA1 | ▲ | ▲ | - | - | - | - | - | - | - | - | - |
| SLC25A3 | - | - | - | - | - | - | - | ▽ | ▽ | - | ▲ |
| SYT11 | - | ▲▽ | - | - | - | - | - | - | - | ▲▽ | - |
| CAPRIN1 | - | ▲ | - | - | - | - | - | - | - | - | ▲ |
| SEPT4 | - | - | - | - | - | - | - | ▲ | ▽ | ▲▽ | - |
| HLA-DRB1 | - | - | ▽ | ▽ | - | - | - | - | - | - | - |
| MST1 | - | ▽ | - | - | - | ▽ | ▽ | - | - | - | - |
| APP | - | - | - | - | ▽ | - | - | ▽ | ▲ | - | - |
| YWHAH | - | - | - | - | ▽ | - | - | - | - | ▲▽ | - |
| PSMB2 | - | - | ▽ | ▽ | - | - | - | - | - | - | - |
| PSMB7 | - | ▽ | - | - | - | - | - | - | - | - | ▽ |
| AGT | - | ▽ | ▽ | - | - | - | - | - | - | - | - |
| TF | - | ▽ | ▽ | - | - | ▲▽ | ▲▽ | - | - | - | - |
Changes in gene expression caused by specific abused substances
Several studies have employed human postmortem brain and gene expression microarrays to examine transcriptional correlates of substance use, including specific use of nicotine, alcohol, cannabis, phencyclidine, cocaine and heroin.17–25,27
Nicotine
In a study of human postmortem hippocampus, smoking was found to produce relatively subtle, but statistically significant changes in gene expression.23 In non-mentally ill control cases, nicotine differentially altered hippocampal gene expression of transcripts functionally associated with cell motility, immune response and the NMDA postsynaptic density. Smoking produced a significant interactive effect on gene expression in the brain of schizophrenic cases, with individual transcripts exhibiting different patterns of expression change across smoking/non-smoking schizophrenic and control cases.
Alcohol
Microarray studies of transcriptional profiles in human postmortem brain, including nucleus accumbens25, motor cortex18, superior frontal cortex17,24, frontal cortex18, and dorsolateral prefrontal cortex25, from alcoholics, have demonstrated gene expression changes for a large array of transcripts, indicating functional changes related to the cytoskeleton, extracellular matrix/cell adhesion molecules, immune/stress response, lipid metabolism, synaptic transmission and intracelullar trafficking, receptors and ion channels, cell signaling, mitochondrial and metabolic processes, protein modification and metabolism, transcriptional and cell cycle regulation.17–18,24–25 A number of these studies have repeatedly found changes, mostly decreases, in the expression of myelin-encoding transcripts.
Cocaine
Examining transcriptional changes in the nucleus accumbens from cocaine abusers identified decreased expression of myelin transcripts for a majority of cases as well as changes in transcripts encoding proteins associated with cytoskeletal and synaptic functions.19 Transcriptional changes in postmortem dorsolateral prefrontal cortex from cocaine abusers indicated altered neural plasticity, oligodendrocyte function, cytoskeleton and related signaling, oxidative phosphorylation, energy metabolism and mitochondrial function.21
Other substance use
Two studies compared transcriptional changes within a brain region for cocaine use and heroin20, cannabis22 and phencyclidine.22 One study by Albertson examined postmortem nucleus accumbens and found significant differences between opioid and cocaine users.20 An increased expression of transcripts associated with synaptic machinery was identified in heroin users, but not for cocaine users. Conversely, a decrease in myelin-related transcripts previously observed for cocaine use cases19 was absent in heroin users20. Interestingly, a few transcripts were significantly and similarly regulated in both groups. Examining differences and similarities across different types of substance use was the premise of another microarray study by our group.22 This study employed postmortem anterior prefrontal cortex from cases who had predominantly used cocaine, cannabis or phencyclidine as determined by case history, hair testing and other toxicology, and found more differences than similarities between drugs in significantly regulated transcripts (Figure 1). A small subset of transcripts did, however, share a significant and similar regulation for cocaine, cannabis and phencyclidine use cases. Functional annotation of the shared transcripts demonstrated a consistent regulation for three functional groups, including a decrease in calcium/calmodulin-related transcripts, and increased expression of Golgi/ER-related transcripts and cholesterol/lipid-related functions, suggesting that altered intracellular trafficking and neuroplasticity may be common functional consequences of cocaine, cannabis and phencyclidine use.
Figure 1. Transcriptional differences and similarities observed for different classes of substance use cases.
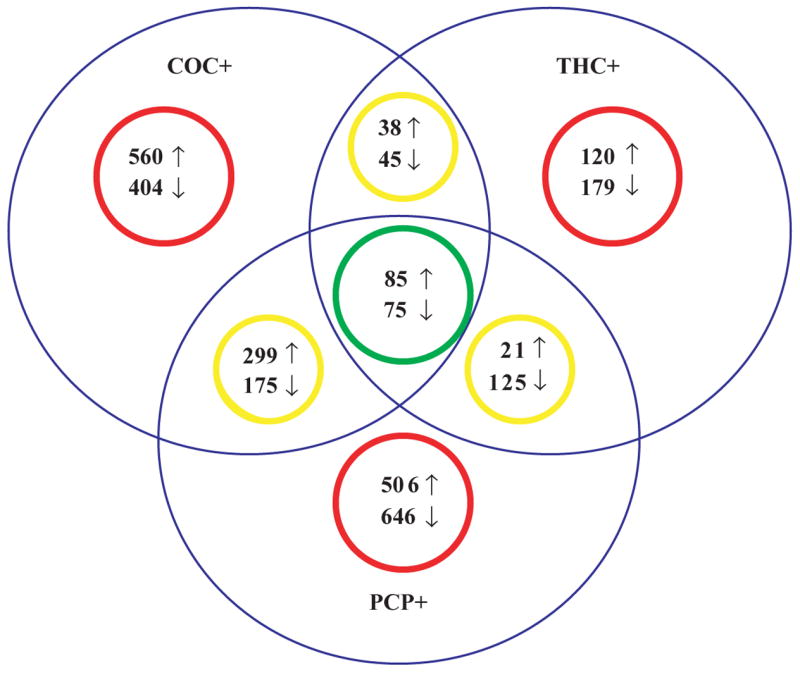
A Venn diagram of significantly changed transcripts detected for substance use cases with both a history (by case history and hair toxicological testing) and a current use (by toxicology) of either cocaine (COC+), cannabis (THC+), or phencyclidine (PCP+) illustrate that there are more differences (red circles) than similarities (green and yellow circles) between different classes of substance use. (Redrawn from illustration in Lehrmann et al., 2006).
Functional implications of common transcriptional changes
As described in the previous sections, functional annotation of transcriptional changes suggest that different abused substances affect a number of similar functional classes, including myelination, synaptic plasticity and cytoskeleton-related functions, while other changes appear to be specific to the type of drug which is abused. While it is important to identify transcripts that are regulated by a specific drug, it is also instructive to look at transcripts regulated across different abused drugs. Additionally, examination of the similarities and differences in the response of different brain regions across these drugs may further provide information pertaining to the regional transcriptional neurobiology. Expression changes were identified for a large number of individual transcripts in nine studies reporting gene lists of significantly changed gene expression in postmortem brain from nicotine, alcohol, cocaine, heroin, cannabis or phencyclidine users.17–23,25,27 Thirty-eight specific gene transcripts were reported in two or more studies (Table 1). These likely represent a conservative estimate of the similarities in genes that are regulated by drugs of abuse, in that different microarray platforms and criteria for significance were employed, and since similar, but not identical, transcripts from the same gene family and functional group were identified in more studies. Among those that were consistently detected, oligodendrocytic function and myelination appeared to be targeted by different classes of drugs of abuse and across brain regions, such that a number of transcripts encoding oligodendrocyte and myelination-related genes were altered in the nucleus accumbens19 and dorsolateral prefrontal cortex21 from cocaine users, and in the nucleus accumbens25 and frontal cortical areas17–18,24–25 from alcoholic cases. Among substance use cases (Table 1), changes in the expression of myelin-associated glycoprotein (MAG) and peripheral myelin protein 22 (PMP22) were exclusively identified in alcoholic cases.17–18,24–25 In contrast, changes in gene expression for proteolipid protein 1 (PLP1), the primary constituent of myelin, was identified to be significantly regulated in the nucleus accumbens19 and dorsolateral prefrontal cortex21 of human cocaine users and in diverse cortical regions in alcoholic cases.17–18,24–25 Changes in PLP1 expression was also identified in schizophrenic cases.29–31 Such changes in oligodendroglial metabolism and function may significantly impact neural communication, including glutamatergic neurotransmission32, and be compounded by transcriptional changes functionally associated with lipid metabolism and function19,21–22,31,33, affecting lipid-mediated synaptic function, signal transduction and intracellular messenger cascades.
As studies of transcriptional regulation in different brain disorders accumulate, so do evidence that some transcripts and functional groups are affected in more than one disorder.29,31,34 These changes may functionally implicate dysregulation of similar neurobiological mechanisms in different disorders. Increased numbers of studies examining and possibly replicating findings from different brain regions and types of substance use are needed to assess these changes and their possible impact on brain function more comprehensively.
Transcriptional subgroups within diagnostic classifications
While substance use and other neuropsychiatric disorders such as schizophrenia and mood disorders are chronic disorders, the presence of a therapeutic or abused substance component may produce dynamic changes in the expression of specific genes. From the temporal changes in gene expression in animal experiments35–36 and from imaging studies of the metabolic responses of the addicted brain to drugs37–39, these changes appear to depend on the nature and manner in which drug(s) are used.
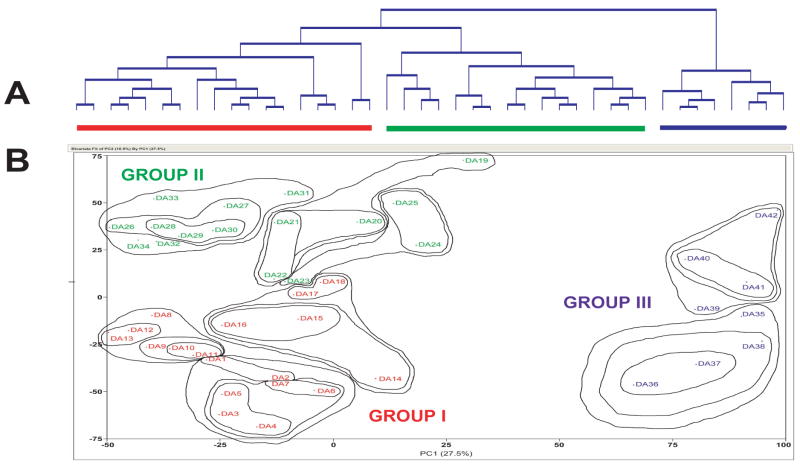
We previously identified a consistent subset of significantly changed transcripts in dorsolateral prefrontal cortex from cocaine users with a history of cocaine use and positive cocaine toxicology at death.21 Two distinct transcriptional patterns were present: For one subgroup that mainly displayed increased expression there was, in addition to cocaine, also toxicological evidence of a smoked crack-cocaine metabolite or of morphine, suggesting that the pattern and/or recency of use differentially affected regulation of this subset of genes. A similar scenario of distinct transcriptional patterns for subgroups was demonstrated in a second study examining postmortem anterior prefrontal cortex from a large cohort of substance use cases.22 Hierarchical clustering and principal components analysis of global gene expression profiles identified three main groups of cases (Figure 2), which did not clearly reflect primary substance use by history and/or toxicology. This was in part due to the presence of significant polysubstance use in Groups I-II, while Group III cases had little or no drugs-of-abuse present at death but had significant other comorbid disease and causes of death. A third example was reported in alcoholic cases with liver cirrhosis.26 The presence of cirrhosis, which often follows and compounds long-term heavy alcohol use, impacted brain gene expression to the extent that specific transcriptional patterns from cirrhotic cases were clearly different from those of non-cirrhotic alcoholics. The authors further noted that where similar transcripts were changed in both the cirrhotic and non-cirrhotic cases, these changes were augmented in cirrhotic cases, and suggested this to reflect a further impairment of normal brain function in cirrhotic cases.
Figure 2. Global transcriptional profiles indicate different transcriptional subtypes.
Hierarchical clustering of global transcriptional profiles from anterior prefrontal cortex from substance use cases22 indicated that three main groups of cases existed (Figure 2. A). This is more clearly visualized in a two-dimensional principal components analysis illustration of the same data (Figure 2. B), with superimposed contour lines encircling nearest neighbors from the hierarchical clustering analysis.
It therefore appears that there can be multiple transcriptional subtypes, “endophenotypes”, within a single classification of users of a specific substance. In other words, within a single diagnostic classification (e.g., cocaine abuse) there may be multiple “endophenotypes” which can be identified by examining changes in gene expression. These transcriptional endophenotypes may be related to the time since last use, the manner and chronicity of use, or be complicated by other comorbid disease21–22,26, including other neuropsychiatric disorders.23,40 Since polysubstance use appears to be the rule, not the exception, in substance users41, gene expression may also be differently impacted by a complex, comorbid use/abuse of multiple drug classes. Additional differences in transcriptional profiles for substance users may arise from comorbid neuropsychiatric disorders, the presence of which has been estimated to range from 10% to 75% depending on the drug of abuse and clinical diagnosis.42–44 Cases belonging to different subtypes within the same diagnostic substance use classification, may exhibit significant differences in gene expression. These differences could result in changes in transcription patterns which are not identified when an entire diagnostic classification (e.g., “cocaine abuse”) is considered. Identification and separate consideration of transcriptional endophenotypes therefore provides a manner in which to capture the full spectrum of transcriptional correlates of substance use neurobiology.
Acknowledgments
This work was supported by the National Institute on Drug Abuse, Intramural Research Program (NIDA IRP), National Institutes of Health (NIH), Department of Health and Human Services (DHHS). We gratefully acknowledge the contributions from our collaborators at the National Institutes of Health: National Institute on Drug Abuse (NIDA): Gloria Gallegos, Zoan R. Afanador, Cellular Neurobiology Research Branch; Ross H. Lowe, W. David Darwin, Jon Oyler, Alan Barnes, Marilyn A. Huestis, Chemistry & Drug Metabolism Section; Jean Lud Cadet, Molecular Neuropsychiatry Research Branch; National Institute of Mental Health (NIMH IRP): Amy Deep-Soboslay, Carlo Colantuoni, Vesna Imamovic, Thomas Hyde, Mary Herman, Joel Kleinman, Clinical Brain Disorders Branch (CBDB), Genes, Cognition and Psychosis (GCAP); National Institute on Aging (NIA IRP): William H. Wood, III, Kevin G. Becker, Gene Expression and Genomics Unit (GEGU), Research Resources Branch (RRB).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Deep-Soboslay A, et al. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57:96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Hill C, et al. Problem of diagnosis in postmortem brain studies of schizophrenia. Am J Psychiatry. 1996;153:533–537. doi: 10.1176/ajp.153.4.533. [DOI] [PubMed] [Google Scholar]
- 5.Lehrmann E, et al. Postmortem diagnosis and toxicological validation of illicit substance use. Addict Biol. 2007 doi: 10.1111/j.1369-1600.2007.00085.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton AJ, et al. Pre- and postmortem influences on brain RNA. J Neurochem. 1993;61:1–11. doi: 10.1111/j.1471-4159.1993.tb03532.x. [DOI] [PubMed] [Google Scholar]
- 7.Hynd MR, et al. Biochemical and molecular studies using human autopsy brain tissue. J Neurochem. 2003;85:543–562. doi: 10.1046/j.1471-4159.2003.01747.x. [DOI] [PubMed] [Google Scholar]
- 8.Leonard S, et al. Biological stability of mRNA isolated from human postmortem brain collections. Biol Psych. 1993;33:456–466. doi: 10.1016/0006-3223(93)90174-c. [DOI] [PubMed] [Google Scholar]
- 9.Lipska BK, et al. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psych. 2006;60:650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Stan AD, et al. Human postmortem tissue: what quality markers matter ? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison PJ, Kleinman JE. Methodological issues. In: Harrison PJ, Roberts GW, editors. The Neuropathology of Schizophrenia. Oxford UP; New York: 2000. pp. 339–350. [Google Scholar]
- 12.Tomita H, et al. Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol Psych. 2004;55:346–352. doi: 10.1016/j.biopsych.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vawter MP, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Mol Psych. 2006;11:663–679. doi: 10.1038/sj.mp.4001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath MC, et al. Variations in respiratory distress characterize the acute agonal period during heroin overdose death: relevance to postmortem mRNA studies. Brain Res Bull. 2006;70:251–259. doi: 10.1016/j.brainresbull.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Galfalvy HC, et al. Sex genes for genomic analysis in human brain: internal controls for comparison of probe level data extraction. BMC Bioinformatics. 2003;4:37. doi: 10.1186/1471-2105-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vawter MP, et al. Gender-specific gene expression in post-mortem human brain: localization to sex chromosomes. Neuropsychopharmacol. 2004;29:373–384. doi: 10.1038/sj.npp.1300337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewohl JM, et al. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- 18.Mayfield RD, et al. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochem. 2002;81:802–8013. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- 19.Albertson DN, et al. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albertson DN, et al. Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacol. 2006;31:2304–2312. doi: 10.1038/sj.npp.1301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehrmann E, et al. Transcriptional profiling in the human prefrontal cortex: evidence for two activational states associated with cocaine abuse. Pharmacogenomics J. 2003;3:27–40. doi: 10.1038/sj.tpj.6500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrmann E, et al. Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS ONE. 2006;1:e114. [Google Scholar]
- 23.Mexal S, et al. Differential modulation of gene expression in the NMDA postsynaptic density of schizophrenic and control smokers. Brain Res Mol Brain Res. 2005;139:317–332. doi: 10.1016/j.molbrainres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacol. 2006;31:1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- 25.Flatscher-Bader T, et al. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. J Neurochem. 2005;93:359–370. doi: 10.1111/j.1471-4159.2004.03021.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, et al. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol Clin Exp Res. 2007;31:1460–1466. doi: 10.1111/j.1530-0277.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, et al. Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J Neurochem. 2004;90:1050–1058. doi: 10.1111/j.1471-4159.2004.02570.x. [DOI] [PubMed] [Google Scholar]
- 28.Hollingshead D, Lewis DA, Mirnics K. Platform influence on DNA microarray data in postmortem brain research. Neurobiol Dis. 2005;18:649–655. doi: 10.1016/j.nbd.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 31.Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol. 2007;10:547–555. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- 32.Tkachev D, et al. Further evidence for altered myelin biosynthesis and glutamatergic dysfunction in schizophrenia. Int J, Neuropsychopharmacol. 2007;10:557–563. doi: 10.1017/S1461145706007334. [DOI] [PubMed] [Google Scholar]
- 33.Mimmack ML, et al. Gene expression analysis in schizophrenia: reproducible up-regulation of several members of the apolipoprotein L family located in a high-susceptibility locus for schizophrenia on chromosome 22. Proc Natl Acad Sci U S A. 2002;99:4680–4685. doi: 10.1073/pnas.032069099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehrmann E, et al. The use of microarrays to characterize neuropsychiatric disorders: postmortem studies of substance abuse and schizophrenia. Curr Mol Med. 2003;3:437–446. doi: 10.2174/1566524033479690. [DOI] [PubMed] [Google Scholar]
- 35.Fagergren P, et al. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- 36.Szumlinski KK, et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacol. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- 37.Breiter HC, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 38.Kufahl PR, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 39.Kosten TR, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacol. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 40.Sokolov BP, et al. Transcription profiling reveals mitochondrial, ubiquitin and signaling abnormalities in postmortem brains from subjects with a history of alcohol abuse or dependence. J Neurosci Res. 2003;72:756–767. doi: 10.1002/jnr.10631. [DOI] [PubMed] [Google Scholar]
- 41.Rounsaville BJ, et al. Single versus multiple drug focus in substance abuse clinical trials research. Drug Alcohol Depend. 2003;70:117–25. doi: 10.1016/s0376-8716(03)00033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conway KP, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- 43.Grant BF, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 44.Swartz MS, et al. Substance use in persons with schizophrenia: baseline prevalence and correlates from the NIMH CATIE study. J Nerv Ment Dis. 2006;194:164–172. doi: 10.1097/01.nmd.0000202575.79453.6e. [DOI] [PubMed] [Google Scholar]