Abstract
While certain circadian hormonal changes are prominent, their predictable assessment requires a standardization of conditions of sampling. The 24-hour rhythm in circulating corticosterone of rodents, known since the 1950s, was studied as a presumed proxy for stress on 108 rats divided into 9 groups of 6 male and 9 groups of 6 female animals sampled every 4 hours for 24 hours. In a first stress study, the “no-rhythm” (zero-amplitude) assumption failed to be rejected at the 5% probability level in the two control groups and in 16 out of the 18 groups considered. A circadian rhythm could be detected with statistical significance, however, in three separate follow-up studies in the same laboratory, each on 168 rats kept on two antiphasic lighting regimens, with 4-hourly sampling for 7 or 14 days. In the first stress study, pooling of certain groups helped the detection and assessment of the circadian corticosterone rhythm. Without extrapolating to hormones other than corticosterone, which may shift more slowly or adjust differently and in response to different synchronizers, the three follow-up studies yielded uncertainty measures (95% confidence intervals) for the point estimate of its circadian period, of possible use in any future study as a reference standard.
The happenstance of a magnetic disturbance at the start of two follow-up studies was associated with the detection of a circasemiseptan component, raising the question whether a geomagnetic disturbance could be considered as a “load”. Far beyond the limitations of sample size, the methodological requirements for standardization in the experimental laboratory concerning designs of studies are considered in the context of models of depression. Lessons from nature's unforeseen geomagnetic contribution and from human studies are noted, all to support the advocacy, in the study of loads, of sampling schedules covering more than 24 hours.
Keywords: Corticosterone, Rhythm, Chronic mild stress, Light shift
Introduction
Longer-than-24-hour sampling is advocated for the consideration of infradian in addition to circadian changes and also to increase the reliability of the estimate of the circadian parameters. It becomes indispensable in looking for desynchronization. Evidence for desynchronization from the environmental routine of the approximate 24-hour rhythms in counts of blood eosinophils and in core temperature had led to the coining of circadian over half a century ago (Halberg et al., 2003a). It seemed appropriate to report incompletely interpretable results in the light of follow-up studies.
Circadian desynchronization of adrenocortical function from the societal routine, specifically with a view of emotional disorders, including mania and depression, was found in data by Bryson and Martin (1954) and was meta-analyzed by Halberg (1960). Rhythms out of sync with their environment continue to be actively investigated (Penev et al. 1998; Van Reeth 1998; Tsai et al., 2005; Bartol-Munier et al., 2006). Focus upon the approximate-weekly (circaseptan) component may bring additional useful information, as suggested by its prominence in negative affect (Cornélissen et al., 2005).
Differences among inbred strains of mice (Halberg and Visscher, 1950) and free-running (for its history, see Halberg et al., 2003a) were indirect hints for a genetic basis of circadian rhythms. Indeed, circadian rhythms are genetically specified (Ralph et al., 1990). Several genes encode proteins essential to clock function, forming an autoregulatory feedsideward loop involving both transcription and translation (Lowrey et al., 2000). The circadian rhythms can be entrained to the external light-dark cycle, but overridden by a magnetic storm (Chibisov et al., 2004; Jozsa et al., 2005a,b). Altered circadian rhythms have been associated with cardiovascular and cerebral disease (Cornélissen et al., 2007), depressive disorders (Halberg, 1968) and shift work (Hennig et al., 1998; see also Minors and Waterhouse 1983; Costa et al., 1989; Florida-James et al., 1996; Davis et al., 2001). Abnormal rhythms at the molecular and cellular levels following stress could represent compromised neural plasticity that contributes to the pathophysiology of stress-induced disorders.
Depressive episodes are often preceded by stressful events, and chronic exposure to stress is an important determinant for both behavioral and neurochemical consequences. The role of stress in depressive disorders lies at the core of several animal models of depression (Katz, 1982; Willner, 1991). Chronomics, the cartography of chronomes, can also be examined for a broader-than-circadian aspect in a budding chronoepidemiology, among others of depression, notably since this condition also relates to stroke and cardiovascular events in a community (Yamanaka et al., 2005) or in individuals with depression (Cornélissen et al., 2005).
Experimental evidence suggests complex relationships between depression and chronic stress. The precise mechanism by which chronic stress (whatever that may mean, since without further qualification the term could stand for long life) may influence rhythms, however, is still not well understood, notwithstanding the thorough study of a few clinical cases that involve the adrenal (Halberg, 1960). Inconsistencies among the findings complicate efforts to model the chronopathology of depression. Glucocorticoids, prime coordinators of both energy balance and stress (Dallman et al., 2002), are influenced by a wide range of variables, including hunger (Dallman et al., 1999), feeding (Hart et al., 1980, Pecoraro et al., 2002), aversive stimuli, expectancy (Hart et al., 1980), “circadian factors” (Halberg et al., 1973; 2003b; Dallman et al., 2002; Pecoraro et al., 2002) and shift-work (Hennig et al., 1998). Chronic mild stress (CMS) in rodents has been proposed to model some of the environmental factors that contribute to the induction of depressive disorders in humans. This model is based on the hypothesis that CMS induces a change in brain reward function that resembles the symptoms of major depression, namely, a decrease in responsiveness to rewarding stimuli.
The CMS model of depression has been criticized in the past for being difficult to replicate. The CMS procedure is associated with a number of neurochemical, neuroendocrine and behavioral effects, like decreased sexual behavior (D'Aquila et al., 1994), increased immobility in the forced swimming test (Molina et al., 1994) and disrupted REM sleep (Cheeta et al., 1997). A repeatedly reported behavioral effect of CMS was assessed via the intake of 1% sucrose (a measure of anhedonia) (Muscat and Willner, 1992; Willner, 1997; Grippo et al., 2003).
While the CMS model has been employed in a variety of paradigms, some of the physiological consequences of this model have, to date, been inconsistent. For instance, studies have reported an elevation of “basal corticosterone levels” in rodents exposed to CMS (Ayensu et al., 1995; Bielajew et al., 2002), whereas others have found biphasic responses (Silberman et al., 2002), no change (Azpiroz et al., 1999) and even decreases in corticosterone following a span of CMS (Murison and Hansen, 2001). Signs of depression are associated with both central and peripheral nervous system alterations. Controversies may arise from ignorance of important rhythm characteristics that are often not inferentially statistically assessed.
Considerations of the recent literature only led to the design of a stress study, aimed at investigating whether, as an animal model of depression, the chronic exposure of unpredictable mild stressors, alone and in combination with an altered environmental lighting schedule, affect the rhythm parameters of certain hormones in rats of both sexes. An insufficient circadian sampling design serves to emphasize the merits of collecting replications with an infradian-circadian scheme. The results in follow-up studies serve to indicate the need to check whether animals have been fully synchronized to the lighting regimen.
Material and Method
Animals and Housing
Locally inbred Wistar rats, 54 male and 54 female (female: 220-250g, male: 300-330 g) were housed in groups of 3 per cage in a climate-controlled environment. During the first 3 weeks, animals were allowed to adjust to their new environment without handling in 12 hours of light (L) (light on from 06:00 to 18:00) alternating with 12 hours of darkness (D) (LD schedule). Thereafter, a mixed factorial design was employed consisting of stress or no-stress × lighting-alteration or no lighting alteration, n=6/group). With the aim to assess the effects of CMS alone, or the effect of a change in lighting regimen alone, or both procedures applied in combination, rats were housed in 5 separate rooms on the following lighting regimens: 1. L06-18D18-06 (LD); 2. LD for 3 days followed by 4 days on reversed LD or DL (D06-18L18-06); 3. LD for 6 days followed by 8 days on DL; 4. LD for 2 days followed by 2 days on DL; 5. LD to keep the control groups of animals in a separate isolated room. Light intensity, at the cage level, was 750 lux. Rats of both sexes were exposed to the same experimental set-up. In rooms 1-4, animals were randomly assigned to one of the treatment conditions: CMS procedure (stressed) and non-stressed, in combination or without any lighting regimen alteration. Table I illustrates the experimental design, with group number assignment. Food and water were available ad libitum in the home cages except for the spans of deprivation specified in the experimental protocol (see below).
Table I.
Study Design
| Room | LD regimen | CMS | Group N |
|---|---|---|---|
| 1 | LD | Yes | 2 |
| No | 6 | ||
| 2 | 3 LD/4 DL | Yes | 3 |
| No | 7 | ||
| 3 | 6 LD/8 DL | Yes | 4 |
| No | 8 | ||
| 4 | 2 LD/2 DL | Yes | 5 |
| No | 9 | ||
| 5 | LD | No | 1 |
LD: L06-18D18-06 (LD)
DL: D06-18L18-06 (DL)
m LD/n DL: m days on LD alternating with n days on DL.
Each group (1-9) includes 6 male and 6 female rats.
Experimental animals were exposed to 4 weeks of chronic stress and/or lighting-regimen alteration, whereas control animals were left undisturbed during this 4-week span, except for scheduled cage cleaning, feeding, and weighing. Sucrose solution (1%) was available ad libitum for a week preceding the experimental procedures to allow for adjustment to the taste. The temperature was maintained at 23 ± 2°C. All animal procedures were done in accordance with and approved by the University of Pecs Institutional Animal Care and Use Committee.
CMS Procedure
Animals assigned to receive chronic mild stress were handled according to the schedule used in a previous report (Grippo et al., 2003). Briefly, during each 1-week span the CMS group was exposed to the following stressors in random order: continuous overnight illumination (one 12-hour span), 40° cage tilt along the vertical axis (two 8-hour spans), paired housing (two 8-hour spans), soiled cage (300 ml water spilled onto bedding; one 16-hour span), an overnight span of water deprivation immediately followed by 1-hour exposure to an empty bottle, stroboscopic illumination (300 flashes/min; one 4-hour span), and white noise (∼90 dB; one 4-hour span of continuous noise). The stressors were presented randomly during a span of 1 week, and then repeated during the weeks thereafter for a total of 4 weeks. Control animals were left undisturbed in their home cages throughout the 4-week span with the exception of general handling (i.e., regular cage cleaning and measuring body weight), which was matched to that of the CMS groups. All rats were decapitated the day after the final day of CMS (day 29). On the day of killing, the planetary index of geomagnetic disturbance (Kp) was 3.125 and the equatorial index of geomagnetic activity (Dst) was 5.
The sucrose consumptions were examined weekly, from 08:00 to 11:00 on Wednesday throughout the experiment. Prior to each test, the control rats (n=12) and the CMS rats (n=48) were food- and water-deprived for 17 hours. During the test span, animals were exposed to two bottles, one containing a 1% sucrose solution and another, water. The bottles were counterbalanced across the left or right side of the cages. Fluid consumption was recorded by reweighing the pre-weighed bottles.
Hormonal Determinations
To measure plasma hormone concentrations, blood samples were collected by heart puncture after cervical dislocation at 4-hour intervals over a 24-hour span starting at 08:00 HALO (hours after light on). The samples were centrifuged to obtain the plasma and stored at -80°C. Plasma corticosterone and testosterone concentrations were determined by radioimmunoassay.
Data Analysis
Means ± standard errors (SEM) were computed. Statistical significance of the differences between the treated groups and the control were evaluated by one-way ANOVA tests and a priori Student's t-tests. A probability value of P <0.05 was considered to be statistically significant. The cosinor method was used to assess the circadian variation. Statistical significance was assessed by means of the zero-amplitude (no-rhythm) test (Halberg 1969; 1980; Cornélissen and Halberg, 2005).
The single cosinor involved the least squares fit of a cosine curve with a given period (here 24 or 12 hours) to each data series, to yield estimates of the MESOR (midline estimating statistic of rhythm, M, a rhythm-adjusted mean value), the double amplitude (2A, a measure of the extent of predictable change within a cycle), and the acrophase (a measure of the timing of overall high values recurring in each cycle). Parameter tests (Bingham et al., 1982) were used to compare the circadian rhythm characteristics among control and stressed groups.
Results
Body Weight
The CMS procedure did not significantly alter body weight relative to control conditions. Body weight during 4 weeks of CMS did not differ between CMS and control groups (P >0.05 for all comparisons).
Sucrose Consumption
The consumption of sucrose solution in the control group was slightly increased during the 4 weeks of the experimental span. In contrast, the consumption in the CMS group was decreased as the stress procedure was performed. On the other hand, the preference for sucrose solution between these two groups showed no statistically significant difference during the 4-week span of the stress procedure. Reference water intake in the CMS group did not differ from the control group's intake following 4 weeks of CMS (P >0.05). A sex difference was observed in each week (week 1: P <0.001, week 2: P <0.001, week 3: P=0.013, week 4: P=0.00l). When comparing the sucrose consumption among lighting regimens, differences were detected starting in the second week (week 2: P=0.004, weeks 3 and 4: P<0.05).
Circadian Hormonal Variation
Plasma concentrations of corticosterone, testosterone and serotonin were measured after 4 weeks of exposure to stress procedures. A circadian rhythm was found to characterize testosterone in the control group (P=0.012). Mean values showed a statistically significant reduction of testosterone of the CMS-treated male rats (n=24) as compared to controls (n=12). A circadian rhythm in serotonin could not be demonstrated in this study with the limited sampling available.
Corticosterone was found to be higher in female than in male rats (P <0.001). When analyzed separately for each group, a circadian rhythm in corticosterone is detected with statistical significance (P <0.05) in only 2 out of 18 analyses. Surprisingly, a circadian rhythm could not be detected in the control group for which the 12-hour component accounted for a larger proportion of the overall variance, albeit without reaching statistical significance either.
In view of the failure to demonstrate a circadian rhythm in corticosterone in the control animals and given the small sample size of each group, the data were pooled across all groups to assess the circadian variation globally. A circadian rhythm of borderline statistical significance is then found to characterize corticosterone in male (P=0.091) but not in female rats. In males, the 12-hour component was more prominent (P=0.026) than the 24-hour variation. A circadian rhythm is again demonstrated for testosterone (P=0.001) but not for serotonin in either males or females.
When data are pooled separately across groups 2-5 (subjected to CMS) and across groups 6-9 (not subjected to CMS), corticosterone in male rats is characterized by a 24-hour component of borderline statistical significance (P=0.095) in the CMS-stressed animals, whereas non-CMS-stressed rats exhibit a 12-hour component of borderline statistical significance (P=0.071). Moreover, a circadian rhythm is demonstrated for testosterone for the CMS-stressed animals (P=0.004) but not for the non-CMS-stressed rats (P >0.20).
Two Kinds of Load: CMS and Changes in LD
Animals in this stress study were exposed to two different kinds of load: the intended CMS and repeated shifts of the lighting regimen. Because schedule shifts are known to disturb circadian rhythmicity, the circadian rhythm characteristics were compared (FIG. 1) between animals kept on the same lighting regimen (groups 1, 2 and 6) and those undergoing shifts in schedule (groups 3-5 and 7-9). Only a small difference in the circadian amplitude of corticosterone was found by parameter tests for female (P=0.085) but not for male rats, animals subjected to repeated shifts of the lighting regimen having a smaller circadian amplitude, as anticipated. Similar results were obtained when considering as controls only animals in groups 2 and 6 (and not those in group 1 which were disturbed once a week for the sucrose test). Again the circadian amplitude of corticosterone of female rats exposed to shifts in lighting regimen was smaller (P=0.030).
FIGURE 1.
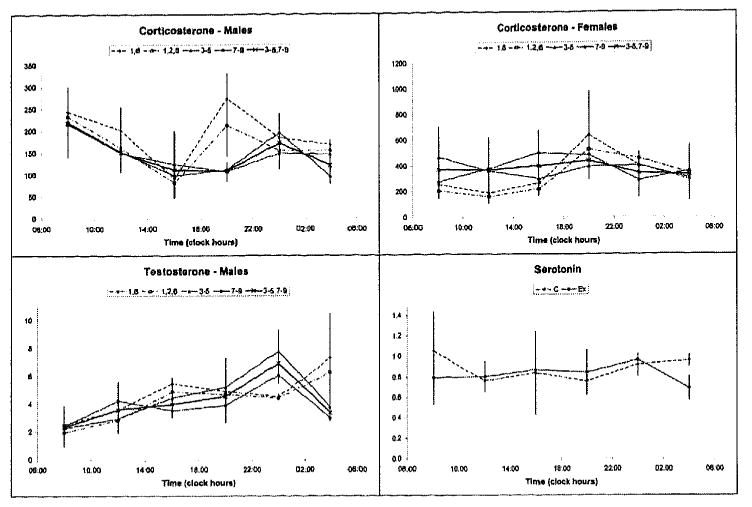
Timepoint means and SEMs of corticosterone, testosterone, and serotonin of animals kept on a fixed (groups 1, 2, 6) or shifting (groups 3-5 and 7-9) lighting regimen.
The observation that no statistically significant difference in circadian rhythm characteristics was found between the CMS-stressed and non-stressed animals (groups 2-5 vs. 6-9 or 3-5 vs. 7-9, including or excluding animals kept on a fixed LD regimen) is a further indication that the load associated with lighting schedule shifts may have been harder than the CMS procedure, at least from the viewpoint of maintaining an integral circadian time structure.
This hypothesis is supported by the extent to which a circadian rhythm in corticosterone can be demonstrated in each group after data expressed as a percentage of mean (to eliminate the sex difference in mean value) were pooled between male and female rats. In groups 2 and 6 kept on a fixed LD regimen, the P-values from the zero-amplitude test were 0.072 and 0.108, respectively; in groups 4 and 8 kept on 8 days of DL alternating with 6 days on LD, spans long enough to allow animals to synchronize to the new routine, the P-values were 0.125 and 0.039, respectively. By contrast, in groups 3 and 7, on a faster-rotating schedule, the P-values were 0.140 and 0.831, respectively, and in groups 5 and 9, on a lighting regimen reversed every other day, the P-values were 0.137 and 0.569, respectively.
Again, groups exposed to CMS seem to be better synchronized than groups not exposed to CMS, suggesting the possibility that some of the loads of the CMS procedure may have acted as added synchronizers of the circadian rhythm. This is also apparent after data are further pooled between CMS-stressed and non-stressed animals kept on the same LD routine. In fixed LD and in 8DL/6LD, the P-values from the zero-amplitude test are 0.021 and 0.005, respectively, but in 4DL/3LD and 2DL/2LD, they are 0.260 and 0.185, respectively.
Discussion
Most functions of living organisms exhibit rhythmic time structures whose characteristics are influenced by cyclic environmental signals. Biological rhythms serve the organism to seek an optimal adjustment to its surroundings. Chronic mild stress is one animal model of depression that is achieved through administration of mild daily stressors over a span of several weeks resulting in decreased consumption of sweetened water (presumably reflecting anhedonia, a core symptom of depression).
The sex difference found for corticosterone is in keeping with an indirectly assessed sex difference revealed by eosinophil counts (Halberg et al., 1957). Regardless of changes due to the gonadal cycle stage, the observed sex difference suggests genetic differences, which influence the mean and amplitude of the rhythm (Touitou and Haus, 2000; Adan and Sanchez-Turet, 2001; Adan and Natale, 2002; Zhao et al., 2003).
One lesson from this study is the need for desirable minimal sampling requirements, even for a rhythm as pronounced as that in serum corticosterone, for which the reversal into antiphase by manipulating the lighting regimen was documented earlier (Halberg et al., 1958). Hence, one faces the choice in models for depression, e.g., between taking more than one animal/timepoint on the same day or replicating the sampling along the 24-hour scale on different days. Given a limited total number of animals, the extension of the study to a week or two is tempting, and was indeed subsequently carried out. Since work every 4 hours for 7 days is fatiguing, each follow-up study was carried out on animals standardized on two antiphasic lighting regimens (Jozsa et al., 2005a,b; Stebelova et al., 2005; Zeman et al., 2005).
Follow-up Studies
Checking that Animals are Synchronized to their Respective Lighting Regimens
During three following surveys, twice for 7 and once for 14 consecutive days, blood and several tissues were collected during daytime working hours only, three times per day at 4-hour intervals from inbred Wistar rats, which had been previously standardized for 1 month in two rooms on antiphasic regimens of 12 hours of light alternating with 12 hours of darkness (LD12:12 and DL12:12). This setup provides a convenient design to study circadian and extracircadian variations over long spans. Sampling actually around the clock a marker rhythm (core temperature) in a subsample of rats validated an about 180° phase difference between rats in the two rooms. A reversed circulating corticosterone rhythm in animals kept in DL, in antiphase with that of rats kept in LD, was also documented in subsamples of 8 animals of each sex sampled around the clock during the first day of the first 7-day follow-up study (FIG. 2). On top, the data are shown in relation to the respective lighting regimens, and at the bottom, they are displayed in hours after light onset (HALO). The findings are in keeping with the proposition that sampling rats at three timepoints 4 hours apart during daytime from two rooms on opposite lighting regimens allows the assessment of circadian changes, the daytime samples from animals kept on the reversed lighting regimen accounting for the samples that would have to be obtained by night from animals kept in the room with the usual lighting regimen.
FIGURE 2.
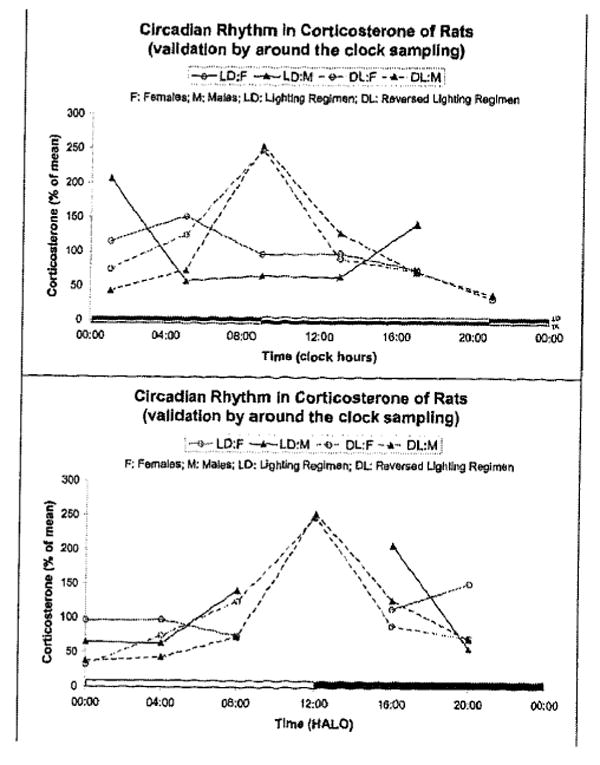
Circadian rhythm in circulating corticosterone in male and female rats kept on two different (antiphasic) lighting regimens. Data from the first 7-day follow-up study during which sampling was done around the clock in both rooms for the first 1.5 days. Data are shown as a function of actual clock hours versus their respective lighting schedule (top) and in hours after light onset (HALO) (bottom).
During the 7-day-long follow-up, circadian and extracircadian spectral components were mapped for serum corticosterone, taking into account the large day-to-day variability. The circadian variation of corticosterone is readily apparent from FIG. 3, as is a sex difference, female rats having higher corticosterone concentrations than male rats, in keeping with results from the CMS study.
FIGURE 3.
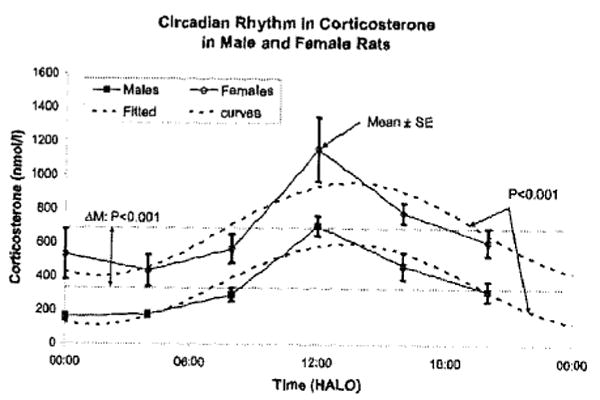
Microscopic demonstration by cosinor of a circadian rhythm in circulating corticosterone of rats kept on antiphasic lighting regimens and sampled for 7 consecutive days. Note sex difference, females having a higher MESOR than males, as ascertained by parameter tests.
Effect of Magnetic Disturbance
The first 7-day follow-up study (April 5-11, 2004) happened to start on the day after the second extremum of a moderate double magnetic storm gauged by the planetary geomagnetic Kp index (which reached 6.3) and by the equatorial index Dst falling to -112 and -81 nT, respectively (FIG. 4). The circadian parameters of circulating corticosterone were more labile during stormy days than during the last three quiet days. In addition to a prominent circadian rhythm, about-weekly (circaseptan) and half-weekly (circasemiseptan) components were also found to characterize corticosterone (Jozsa et al., 2005a,b). The concomitantly assessed circadian melatonin rhythm was also altered, the MESOR and circadian amplitude being decreased for pineal melatonin but increased for hypothalamic melatonin (FIG. 4). Recording calendar dates of experiments and consulting forecasts and/or nowcasts is recommended in planning studies and reporting results in order to enable the further investigation of the role of magnetic storms as a putative load.
FIGURE 4.
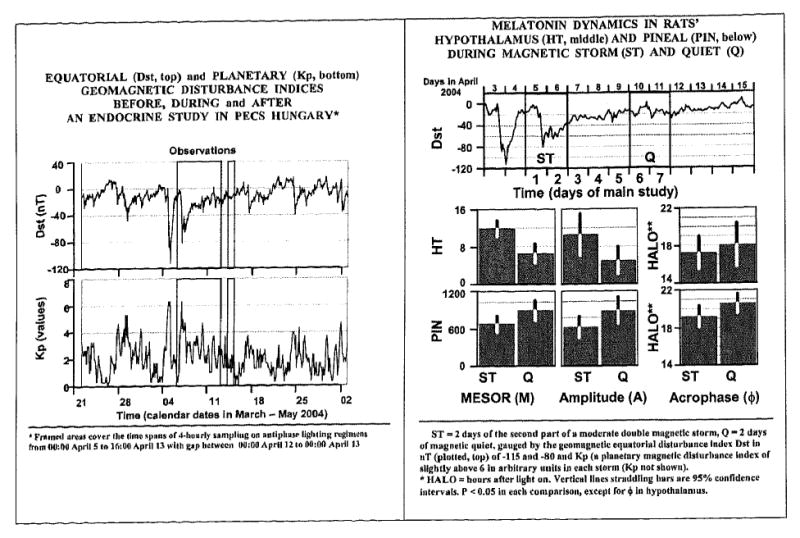
Magnetic storms, gauged by two geomagnetic indices (left), may constitute a load, as suggested by alterations in the circadian rhythm of pineal and hypothalamic melatonin in rats sampled for 7 days in two antiphasic lighting regimens. An increased day-to-day variability in the circadian parameters of circulating corticosterone during magnetically disturbed vs quiet days (not shown) was also noted in this follow-up study.
Effect of Food Deprivation
In a second 7-day follow-up study, the effect of food deprivation was a testable load. An elevation in corticosterone associated with starvation, a load (or “stressor” if “stress” is used as a synonym for “strain”), was statistically significant in male rats (FIG. 5), but not in female rats. A sex difference in corticosterone was again found, in keeping with results observed herein.
FIGURE 5.
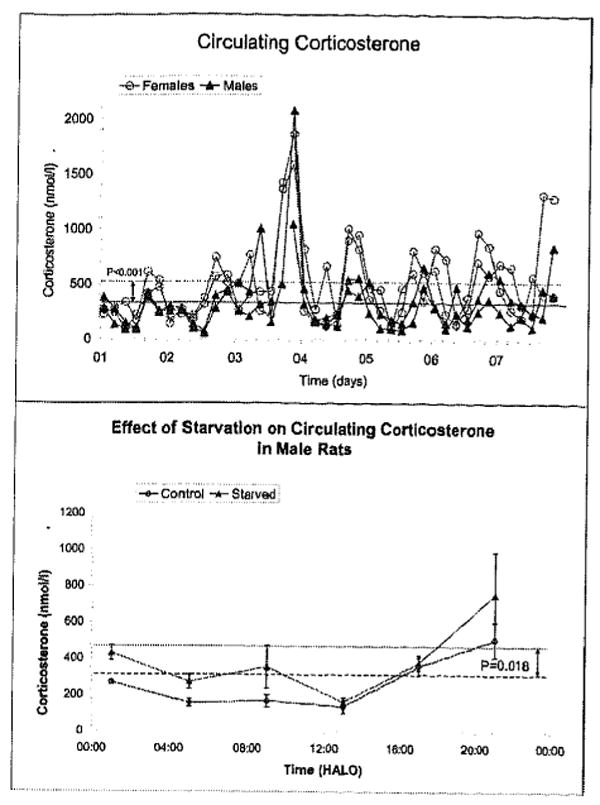
Effect of food deprivation on circulating corticosterone of rats kept on antiphasic lighting regimens, sampled for 7 consecutive days. Female rats have higher concentrations than male rats (top). Food deprivation is also associated with elevated concentrations of corticosterone in male rats (bottom) but not in female rats (not shown).
Robustness of the Circadian Corticosterone Rhythm During Geomagnetic Quiet
A third follow-up study lasting 14 days allowed best the provision of uncertainties of the circadian period estimate for corticosterone (Table II). It also corroborated the sex difference observed in all other studies reviewed herein.
Table II.
Estimate of 24-h synchronized circadian period of circulating corticosterone in Wistar rats of both sexes*
| Group | Period (hours) (95% CI) | Amplitude (95% CI) | |||||
| Original data (nmol/l) All | 24.036 | (23.60, | 24.48) | 366.28 | (205.75, | 526.81) | |
| Females | 23.987 | (23.51, | 24.47) | 525.17 | (271.25, | 779.09) | |
| Males | 24.131 | (23.59, | 24.67) | 223.50 | (102.71, | 344.28) | |
| Females: | week 1 | 23.890 | (22.30, | 25.48) | 561.94 | (124.78, | 999.10) |
| ” 2 | 23.600 | (22.51, | 24.69) | 506.95 | (203.41, | 810.50) | |
| Males: | week 1 | 25.492 | (24.12, | 26.87) | 277.73 | (98.52, | 456.94) |
| ” 2 | 23.546 | (22.30, | 24.79) | 235.98 | (75.22, | 396.73) | |
| Log10- transformed data | |||||||
| All | 24.084 | (23.74, | 24.43) | 0.28 | (0.18, | 0.37) | |
| Females | 24.110 | (23.69, | 24.53) | 0.31 | (0.18, | 0.44) | |
| Males | 24.023 | (23.59, | 24.46) | 0.26 | (0.15, | 0.37) | |
| Females: | week 1 | 23.943 | (22.48, | 25.41) | 0.30 | (0.09, | 0.51) |
| ” 2 | 23.679 | (22.74, | 24.62) | 0.33 | (0.16, | 0.49) | |
| Males: | week 1 | 25.086 | (23.95, | 26.22) | 0.29 | (0.14, | 0.44) |
| ” 2 | 23.588 | (22.45, | 24.73) | 0.26 | (0.10, | 0.43) | |
Computed by extended linear-nonlinear cosinors (Marquardt 1963; Halberg 1980; Cornélissen and Halberg, 2005) in data from Wistar rats sampled at 4-hour intervals for 2 weeks after over 1-month standardization in antiphasic regimens of light and darkness alternating at 12-hour intervals (for standardization see Jozsa et al. 2005a,b).
Limitations
The stress study suffered some limitations. The number of replications was in retrospect insufficient, notwithstanding the usual robust large-amplitude circadian rhythm of corticosterone. This is primarily apparent from the failure to demonstrate a circadian rhythm in the control group kept mostly undisturbed for 4 weeks on the same lighting regimen. It may have been a mistake to examine sucrose consumption in these animals in a fashion which required depriving them of food and water for 17 hours once a week.
Conclusions
The present study suggests that changing lighting regimens may have been more of a load than the CMS procedure, at least from the viewpoint of the integrity of the circadian system of corticosterone.
Concomitant circadian and infradian exploration is recommended for studies of corticosterone in order to obtain 95% confidence intervals for the point estimate of the period.
Rhythms, as statistical entities, obey inferential rules. Hence sampling designs are best based on replications that can be transverse, longitudinal or hybrid. A single equidistant N-of-6 per cycle design serves at best for exploration and is not invariably satisfactory. Moreover, one may alter, and occasionally even fail to detect a rhythm, when a room is entered repeatedly.
A 7-day design, happening to fall on a day of a magnetic storm, may have gauged a special feature of a load (stress), including a single stimulus-associated manifestation of a circasemiseptan component in circulating corticosterone, in association with a magnetic storm. This observation suggests the importance of checking for the occurrence of geomagnetic disturbances that should be taken into account when effects of any loads (stress) on hormonal rhythm parameters are being studied.
Acknowledgments
Supported by ETT 314/2006, Hungary; NIH GM-13981 (FH), and University of Minnesota Supercomput-ing Institute (GC, FH).
References
- Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19:709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Adan A, Sanchez-Turet M. Gender differences in diurnal variations of subjective activation and mood. Chronobiol Int. 2001;18(3):491–502. doi: 10.1081/cbi-100103971. [DOI] [PubMed] [Google Scholar]
- Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS. Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol Behav. 1995;57:165–169. doi: 10.1016/0031-9384(94)00204-i. [DOI] [PubMed] [Google Scholar]
- Azpiroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferation response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology. 1999;24:345–361. doi: 10.1016/s0306-4530(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Bartol-Munier I, Gourmelen S, Pevet P, Challet E. Combined effects of high-fat feeding and circadian desynchronization. Int J Obesity. 2006;30:60–67. doi: 10.1038/sj.ijo.0803048. [DOI] [PubMed] [Google Scholar]
- Bielajew C, Konkle AT, Merali Z. the effects of chronic mild stress on male Sprague-Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behav Brain Res. 2002;136(2):583–592. doi: 10.1016/s0166-4328(02)00222-x. [DOI] [PubMed] [Google Scholar]
- Bingham C, Arbogast B, Cornélissen Guillaume G, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- Bryson RW, Martin DF. 17-Ketosteroid excretion in a case of manic-depressive psychosis. The Lancet. 1954;267:365–367. doi: 10.1016/s0140-6736(54)92667-7. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Ruigt G, van Proosdij J, Willner P. Changes in sleep architecture following chronic mild stress. Biol Psychiatr. 1997;41:419–427. doi: 10.1016/S0006-3223(96)00058-3. [DOI] [PubMed] [Google Scholar]
- Chibisov SM, Cornélissen G, Halberg F. Magnetic storm effect on the circulation of rabbits. Biomed Pharmacother. 2004;58(Suppl 1):S15–S19. doi: 10.1016/s0753-3322(04)80003-9. [DOI] [PubMed] [Google Scholar]
- Cornélissen G, Halberg F. Chronomedicine. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. 2nd. John Wiley & Sons Ltd; Chichester, UK: 2005. pp. 796–812. [Google Scholar]
- Cornélissen G, Watson D, Mitsutake G, Fiser B, Siegelova J, Dusek J, Vohlidalova L, Svacinova H, Halberg F. Mapping of circaseptan and circadian changes in mood. Scripta Medica. 2005;78:89–98. [PMC free article] [PubMed] [Google Scholar]
- Cornélissen G, Halberg F, Otsuka K, Singh RB, Chen CH. Chronobiology predicts actual and proxy outcomes when dipping fails. Hypertension. 2007;49:237–239. doi: 10.1161/01.HYP.0000250392.51418.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G, Lievore F, Casaletti G, Gaffuri E, Folkard S. Circadian characteristics influencing individual differences in tolerances and adjustment to shift work. Ergonomics. 1989;32:373–385. doi: 10.1080/00140138908966104. [DOI] [PubMed] [Google Scholar]
- D'Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Viau V, Bhatnagar S, Gomez F, Laugero K, Bell ME. Corticotropin-releasing factor, corticosteroids, and sugar: energy balance, the brain, and behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrback SE, Rubin RT, editors. Mammalian Hormone Systems. Academic Press; San Diego, CA: 2002. pp. 571–632. [Google Scholar]
- Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and the risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- Florida-James G, Wallymahmed A, Reilly T. Effects of nocturnal shiftwork on mood states of student nurses. Chronobiol Int. 1996;13(1):59–69. doi: 10.3109/07420529609040842. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- Halberg F. Symposium on “Some current research methods and results with special reference to the central nervous system”. Physiopathologic approach. Am J Ment Defic. 1960;65:156–171. [PubMed] [Google Scholar]
- Halberg F. Circadian desynchronization. In: Fomon SJ, editor. Circadian Systems; Report of the 39th Ross Conference on Pediatric Research; Columbus, OH: Ross Laboratories; 1961. pp. 18–19. [Google Scholar]
- Halberg F. Symposium Bel-Air III. Cycles biologiques et psychiatrie / publié sous la direction du professeur J de Ajuriaguerra. Geneva: Georg; Paris: Masson et Cle; 1968. Physiologic considerations underlying rhythmometry, with special reference to emotional illness. Symposium on Biological Cycles and Psychiatry; pp. 73–126. [Google Scholar]
- Halberg F. Chronobiology. Annu Rev Physiol. 1969;31:675–725. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- Halberg F. Chronobiology: methodological problems. Acta Med Rom. 1980;18:399–440. [Google Scholar]
- Halberg F, Visscher MB. Regular diurnal physiological variation in eosinophil levels in five stocks of mice. Proc Soc Exp Biol. 1950;75:846–847. doi: 10.3181/00379727-75-18365. [DOI] [PubMed] [Google Scholar]
- Halberg F, Visscher MB, Bittner JJ. Eosinophil rhythm in mice. Range of occurrence; effects of illumination, feeding and adrenalectomy. Am J Physiol. 1953;174:109–122. doi: 10.1152/ajplegacy.1953.174.1.109. [DOI] [PubMed] [Google Scholar]
- Halberg F, Hamerston O, Bittner JJ. Sex difference in eosinophil counts in tail blood of mature B1 mice. Science. 1957;125:73. doi: 10.1126/science.125.3237.73. [DOI] [PubMed] [Google Scholar]
- Halberg F, Barnum CP, Silber RH, Bittner JJ. 24-hour rhythms at several levels of integration in mice on different lighting regimens. Proc Soc Exp Biol. 1958;97:897–900. doi: 10.3181/00379727-97-23915. [DOI] [PubMed] [Google Scholar]
- Halberg F, Haus E, Cardoso SS, Scheving LE, Kühl JFW, Shiotsuka R, Rosene G, Pauly JE, Runge W, Spalding JF, Lee JK, Good RA. Toward a chronotherapy of neoplasia: tolerance of treatment depends upon host rhythms. Experientia (Basel) 1973;29:909–934. doi: 10.1007/BF01930381. [DOI] [PubMed] [Google Scholar]
- Halberg F, Cornélissen G, Katinas G, Syutkina EV, Sothern RB, Zaslavskaya R, Halberg F, Watanabe Y, Schwartzkopff O, Otsuka K, Tarquini R, Perfetto P, Siegelova J. Transdisciplinary unifying implications of circadian findings in the 1950s. J Circadian Rhythms. 2003a;1(2):61. doi: 10.1186/1740-3391-1-2. www.JCircadianRhythms.com/content/pdf/1740-3391-2-3.pdf. [DOI] [PMC free article] [PubMed]
- Halberg F, Cornélissen G, Spector NH, Sonkowsky RP, Otsuka K, Baciu I, Hriscu M, Schwartzkopff O, Bakken EE. Stress/strain/life revisited. Quantification by blood pressure chronomics: benetensive, transtensive or maletensive chrono-vasculo-neuro-immuno-modulation. Biomed Pharmacother. 2003b;57(Suppl 1):136s–163s. doi: 10.1016/j.biopha.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Hart RP, Coover GD, Shnerson A, Smotherman WP. Plasma corticosterone elevations in rats in response to consumption of concentrated sugar solutions. J Comp Physiol Psychol. 1980;94:337–345. doi: 10.1037/h0077662. [DOI] [PubMed] [Google Scholar]
- Hennig J, Kieferdorf P, Moritz C, Huwe S, Netter P. Changes in Cortisol secretion during shiftwork: implications for tolerance to shiftwork? Ergonomics. 1998;41(5):610–621. doi: 10.1080/001401398186784. [DOI] [PubMed] [Google Scholar]
- Jozsa R, Halberg F, Cornélissen G, Zeman M, Kazsaki J, Csernus V, Katinas GS, Wendt HW, Schwartzkopff O, Stebelova K, Dulkova K, Chibisov SM, Engebretson M, Pan W, Bubenik GA, Nagy G, Herold M, Hardeland R, Hüther G, Pöggeler B, Tarquini R, Perfetto F, Salti R, Olah A, Csokas N, Delmore P, Otsuka K, Bakken EE, Allen J, Amory-Mazaudier C. Chronomics, neuroendocrine feedsidewards and the recording and consulting of nowcasts forecasts of geomagnetics. Biomed Pharmacother. 2005a;59(Suppl 1):S24–S30. doi: 10.1016/s0753-3322(05)80006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsa R, Olah A, Cornélissen G, Csernus V, Otsuka K, Zeman M, Nagy G, Kazsaki J, Stebelova K, Csokas N, Pan W, Herold M, Bakken EE, Halberg F. Circadian and extracircadian exploration during daytime hours of circulating corticosterone and other endocrine chronomes. Biomed Pharmacother. 2005b;59(Suppl 1):S109–S116. doi: 10.1016/s0753-3322(05)80018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RJ, Siebel M. Animal model of depression: tests of three structurally and pharmacologically novel antidepressant compounds. Pharmacol Biochem Behav. 1982;16:973–977. doi: 10.1016/0091-3057(82)90055-7. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Youngstedt SD, Elliott JA et al. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22:695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt DW. An algorithm for least-squares estimation of nonlinear parameters. J Soc Indust Appl Math. 1963;11:431–441. [Google Scholar]
- Minors DS, Waterhouse JM. Circadian rhythm amplitude - is it related to rhythm adjustment and/or worker motivation? Ergonomics. 1983;26:229–241. doi: 10.1080/00140138308963338. [DOI] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in forced swim test: reversal by naloxone. Psychopharmacol. 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- Murison R, Hansen AL. Reliability of the chronic mild stress paradigm: implications for research and animal welfare. Integr Physiol Behav Sci. 2001;36:266–274. [Google Scholar]
- Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev. 1992;16:507–517. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- Olah A, Jozsa R, Cornélissen G, Csernus V, Zeman M, Nagy G, Pan WH, Hoogerwerf WA, Kazsaki J, Otsuka K, Wang ZR, Sothern RB, Sothern SB, Halberg F. Sampling for chronomics extended circadian phase map of the laboratory rat. Proc. Intl. Conf. Frontiers of Biomed. Sci.: Chronobiol.; Chengdu, China. 2006. pp. 46–49. [Google Scholar]
- Pecoraro N, Gomez F, Laugero K, Dallman MF. Brief access to sucrose engages food - entrainable rhythms in food-deprived rats. Behav Neurosci. 2002;116:757–776. [PubMed] [Google Scholar]
- Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275(6 Pt 2):H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Schweiger HG, Berger S, Kretschmer H, Mörler H, Halberg E, Sothern RB, Halberg F. Evidence for a circaseptan and a circasemiseptan growth response to light/dark cycle shifts in nucleated and enucleated Acetabularia cells, respectively. Proc Natl Acad Sci USA. 1986;83:8619–8623. doi: 10.1073/pnas.83.22.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman DM, Wald M, Genaro AM. Effects of chronic mild stress on lymphocyte proliferative response. Participation of serum thyroid hormones and corticosterone. Int Immunopharmacol. 2002;2:487–497. doi: 10.1016/s1567-5769(01)00190-4. [DOI] [PubMed] [Google Scholar]
- Stebelova K, Zeman M, Cornélissen G, Bubenik G, Jozsa R, Hardeland R, Poeggeler B, Huether G, Olah A, Nagy G, Csernus V, Kazsaki J, Pan W, Otsuka K, Bakken EE, Halberg F. Chronomics reveal and quantify circadian rhythmic melatonin in duodenum of rats. Biomed Pharmacother. 2005;59(Suppl 1):S209–S212. doi: 10.1016/s0753-3322(05)80033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquini B, Cornélissen G, Perfetto F, Tarquini R, Halberg F. Chronome assessment of circulating melatonin in humans. In vivo. 1997;11:473–484. [PubMed] [Google Scholar]
- Touitou Y, Haus E. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol Int. 2000;17:369–390. doi: 10.1081/cbi-100101052. [DOI] [PubMed] [Google Scholar]
- Tsai LL, Tsai YC, Hwang K, Hwang YW, Tzeng JE. Repeated light-dark shifts speed up body weight gain in male F344 rats. Am J Physiol Endocrinol Metab. 2005;289(2):E212–E217. doi: 10.1152/ajpendo.00603.2004. [DOI] [PubMed] [Google Scholar]
- Van Reeth O. Sleep and circadian disturbances in shift-work: strategies for their management. Hormone Res. 1998;49:158–162. doi: 10.1159/000023164. [DOI] [PubMed] [Google Scholar]
- Willner P, Sampson D, Papp M, Phillips G, Muscat R. Animal models of anhedonia, anxiety, depression and mania. In: Soubrie P, editor. Animal Models of Psychiatric Disorders. Karger; Basel: 1991. pp. 71–99. [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacot (Berl.) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Yamanaka G, Otsuka K, Hotta N, Murakami S, Kubo Y, Matsuoka O, Takasugi E, Yamanaka T, Shinagawa M, Nunoda S, Nishimura Y, Shibata K, Saitoh H, Nishinaga M, Ishine M, Wada T, Okumiya K, Matsubayashi K, Yano S, Ishizuka S, Ichihara K, Cornélissen G, Halberg F. Depressive mood is independently related to stroke and cardiovascular events in a community. Biomed Pharmacother. 2005;59(Suppl 1):S31–S39. doi: 10.1016/s0753-3322(05)80007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman M, Jozsa R, Cornélissen G, Stebelova K, Bubenik G, Olah A, Poeggeler B, Huether G, Hardeland R, Nagy G, Csernus V, Pan W, Otsuka K, Halberg F. Chronomics: circadian lead of extrapineal vs. pineal melatonin rhythms with an infradian hypothalamic exploration. Biomed Pharmacother. 2005;59(Suppl 1):S213–S219. doi: 10.1016/s0753-3322(05)80034-4. [DOI] [PubMed] [Google Scholar]
- Zhao ZY, Xie Y, Fu YR, Li YY, Bogdan A, Touitou Y. Circadian rhythm characteristics of serum Cortisol and dehydroepiandrosterone sulfate in healthy Chinese men aged 30 to 60 years. A cross-sectional study. Steroids. 2003;68(2):133–138. doi: 10.1016/s0039-128x(02)00167-8. [DOI] [PubMed] [Google Scholar]


