Abstract
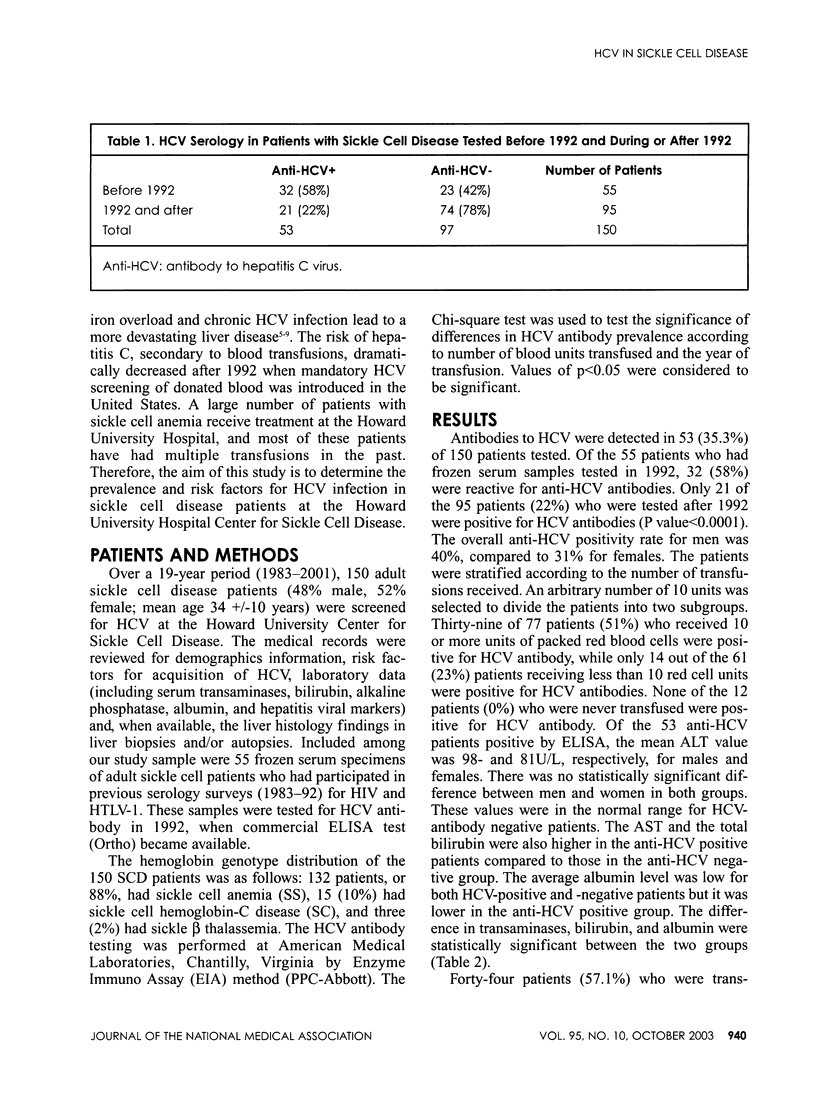
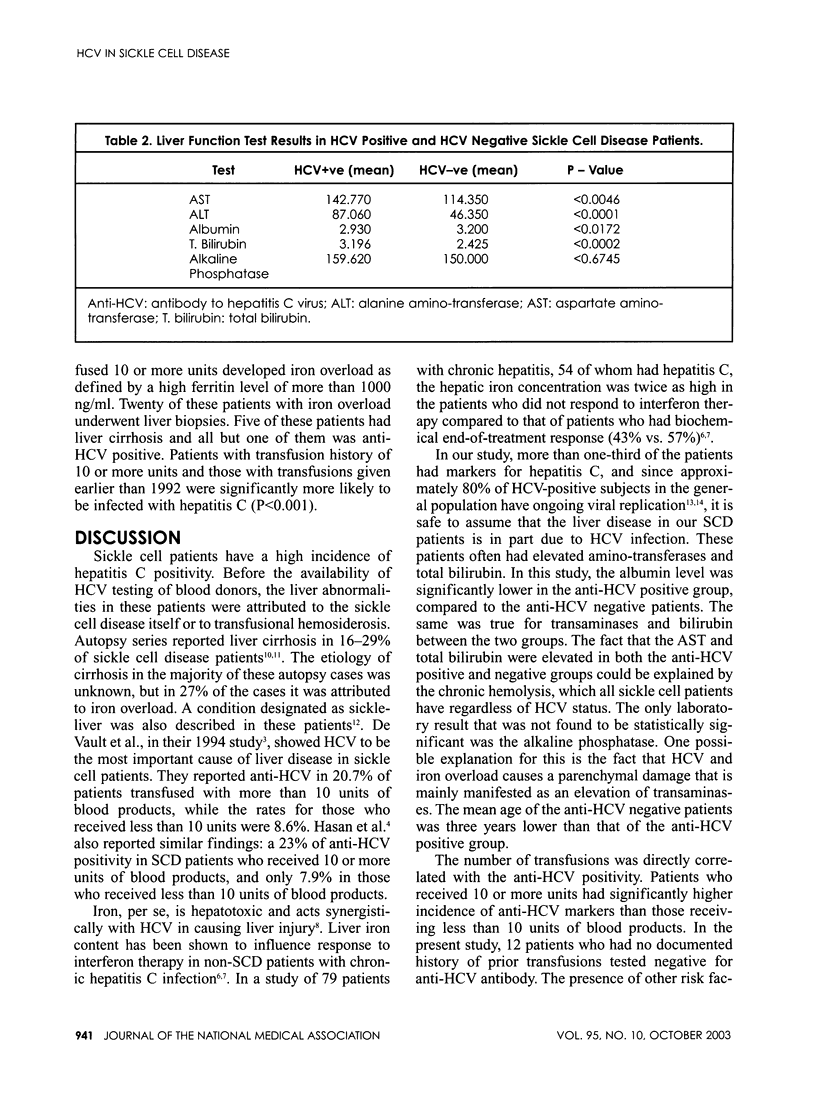
PURPOSE: To determine the prevalence of hepatitis C virus antibodies (anti-HCV) in patients with sickle cell disease. PATIENTS AND METHODS: Between 1983 and 2001, 150 patients from the Howard University Hospital Center for Sickle Cell Disease were screened for HCV antibody (52% women, 48% men, mean age 34 years). Frozen serum samples from 56 adult sickle cell patients who had participated in previous surveys (1983-92) of HIV and HTLV-1 serology and who were tested in 1992 for anti-HCV antibody--when commercial ELISA test (Ortho) became available--were included in this paper. Of the 150 patients in the study, 132 had sickle cell anemia genotype (SS), 15 had sickle cell hemoglobin-C disease (SC) and three had sickle beta thalassemia. Clinical charts were reviewed for history of blood transfusion, IV drug abuse, homosexuality, tattooing, iron overload, and alcohol abuse. RESULTS: Antibodies to HCV were detected in 53 patients (35.3%). Of the 55 patients who had frozen serum samples tested in 1992, 32 (58%) were reactive for anti-HCV, while only 21 of the 95 patients (22%) tested after 1992 were positive for HCV antibodies (P<0.001). Thirty-nine of 77 patients (51%) who received more than 10 units of packed red blood cells were positive for HCV antibody, and only 14 of 61 patients (23%) who received less than 10 units of packed red blood cells transfusion were positive for HCV antibodies (P<0.001). None of the 12 patients who never received transfusion were positive for HCV antibody. In the 53 anti-HCV positive patients, the mean alanine amino-transferase (ALT) value was 98- and 81 U/L, respectively, for males and females. These values were normal for the HCV-antibody negative patients. The aspartate amino-transferase (AST) and the total bilirubin were also higher in the anti-HCV positive patients compared to patients in the anti-HCV negative group. Forty-four patients (57.1%) who were transfused more than 10 units developed iron overload defined by a serum ferritin level higher than 1,000 ng/ml. A total of 20 of the patients with iron overload underwent liver biopsies. Seven of these 20 patients (35%) were HCV positive. These patients often had more severe liver disease and higher degree of iron deposition. CONCLUSION: The prevalence of HCV antibody and iron overload is directly related to the number of blood transfusions in patients with sickle cell disease. The prevalence of HCV infection has decreased significantly, since blood donor screening for HCV became available. Chronic HCV infection and iron overload place sickle cell patients at risk for significant liver disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Seeff L. B. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- Bauer T. W., Moore G. W., Hutchins G. M. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med. 1980 Dec;69(6):833–837. doi: 10.1016/s0002-9343(80)80008-8. [DOI] [PubMed] [Google Scholar]
- DeVault K. R., Friedman L. S., Westerberg S., Martin P., Hosein B., Ballas S. K. Hepatitis C in sickle cell anemia. J Clin Gastroenterol. 1994 Apr;18(3):206–209. doi: 10.1097/00004836-199404000-00006. [DOI] [PubMed] [Google Scholar]
- Fong T. L., Han S. H., Tsai N. C., Morgan T. R., Mizokami M., Qian D., Phan C., Goad K., Redeker A. G. A pilot randomized, controlled trial of the effect of iron depletion on long-term response to alpha-interferon in patients with chronic hepatitis C. J Hepatol. 1998 Mar;28(3):369–374. doi: 10.1016/s0168-8278(98)80308-5. [DOI] [PubMed] [Google Scholar]
- GREEN T. W., CONLEY C. L., BERTHRONG M. [The liver in sickle cell anemia]. Bull Johns Hopkins Hosp. 1953 Feb;92(2):99–127. [PubMed] [Google Scholar]
- Hasan M. F., Marsh F., Posner G., Bellevue R., Dosik H., Suatengco R., Ramani N. Chronic hepatitis C in patients with sickle cell disease. Am J Gastroenterol. 1996 Jun;91(6):1204–1206. [PubMed] [Google Scholar]
- Ikura Y., Morimoto H., Johmura H., Fukui M., Sakurai M. Relationship between hepatic iron deposits and response to interferon in chronic hepatitis C. Am J Gastroenterol. 1996 Jul;91(7):1367–1373. [PubMed] [Google Scholar]
- Price D. T., Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999 Jul;107(1):85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- Roeckel I. E. Commentary: Iron metabolism in hepatitis C infection. Ann Clin Lab Sci. 2000 Apr;30(2):163–165. [PubMed] [Google Scholar]
- Rosenblate H. J., Eisenstein R., Holmes A. W. The liver in sickle cell anemia. A clinical-pathologic study. Arch Pathol. 1970 Sep;90(3):235–245. [PubMed] [Google Scholar]
- SONG Y. S. Hepatic lesions in sickle cell anemia. Am J Pathol. 1957 Mar-Apr;33(2):331–351. [PMC free article] [PubMed] [Google Scholar]
- Troisi C. L., Hollinger F. B., Hoots W. K., Contant C., Gill J., Ragni M., Parmley R., Sexauer C., Gomperts E., Buchanan G. A multicenter study of viral hepatitis in a United States hemophilic population. Blood. 1993 Jan 15;81(2):412–418. [PubMed] [Google Scholar]


