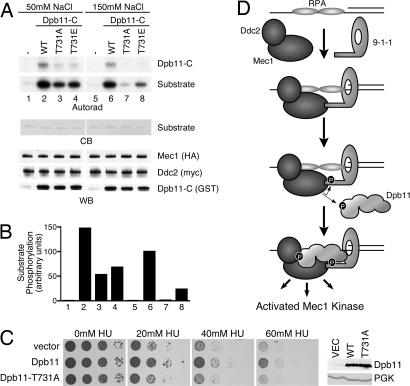
Fig. 5.
Dpb11 T731 phosphorylation potentiates Mec1 activation. (A) Mec1–Ddc2 complexes were purified and incubated with wild-type or mutant forms of Dpb11-C, substrate, and γ-32P ATP. Kinase reactions were separated by SDS/PAGE, stained with Coomassie blue (CB) to visualize the substrate, and exposed to film (autorad). A duplicate gel was blotted and probed with anti-HA and anti-myc antibodies to detect Mec1 and Ddc2, respectively. An anti-GST antibody was used to detect Dpb11-C proteins (WB). (B) Quantification of substrate phosphorylation corresponding to the numbered lanes in A. (C) YDM003, a Δddc2 Δsml1 yeast strain carrying pDM158 (a URA3 CEN plasmid expressing ddc2-top under its endogenous promoter) was transformed with galactose-inducible Dpb11 or Dpb11 T731A (2μ) expression vectors or an empty vector control. Cells were grown to mid-log phase and serial dilutions were spotted onto galactose plates with the indicated concentration of hydroxyurea (HU) and incubated at 30 °C. The expression level of the wild-type and T731A Dpb11 proteins was confirmed to be equivalent by Western blotting. Anti-PGK antibodies were used as a loading control. (D) Simplified model of Mec1–Ddc2 activation described in Discussion. In this model, we propose that Dpb11 functions downstream of 9-1-1. Our data do not exclude the possibility that Dpb11 could function upstream of 9-1-1 in some circumstances. In this alternative scenario, an initial weak Dpb11-Ddc2 interaction could promote partial Mec1 activation, leading to Ddc1 phosphorylation. Phosphorylated Ddc1 could then stabilize the Dpb11 protein at the damaged site and allow further amplification of Mec1 signaling.