Abstract
How glucose levels affect bone in patients with primary hyperparathyroidism is unknown, although the prevalence of impaired glucose metabolism is higher in patients with primary hyperparathyroidism. The present study was performed to examine the relationships between fasting plasma glucose (FPG) and various indices in 93 postmenopausal women with primary hyperparathyroidism. Bone mineral density (BMD) and body composition were measured by dual-energy Xray absorptiometry. Body weight, body mass index (BMI), fat mass and % fat were positively related to FPG. Serum levels of calcium and parathyroid hormone (PTH) as well as bone metabolic indices were not related to FPG and immunoreactive insulin levels. As for BMD, FPG was positively related to the Z scores of BMD at the lumbar spine and femoral neck, although it was not significantly related to the Z-score of BMD at the radius. On the other hand, immunoreactive insulin levels were not significantly related to BMD parameters at any sites. In multiple regression analysis, FPG was significantly related to BMD (Z score) at the lumbar spine and femoral neck, when body weight, BMI, immunoreactive insulin, PTH, and bone resorption indices were considered; however, these relationships at the lumbar spine were not significant when fat mass was considered. In conclusion, the present study indicated that FPG levels were positively related to BMD at the lumbar spine and femoral neck in postmenopausal women with primary hyperparathyroidism.
Keywords: Primary hyperparathyroidism, glucose, bone mineral density, body composition
Introduction
In primary hyperparathyroidism, bone metabolism is affected by excessive secretion of parathyroid hormone (PTH) from the parathyroid glands. Patients with primary hyperparathyroidism have reduced bone mineral density (BMD), especially at the cortical bone with increased bone turnover [1–3]. In our previous study, bone mass was reduced in primary hyperparathyroidism patients at both cortical and trabecular bones by forearm volumetric BMD analysis with peripheral quantitative computed tomography in female subjects [4, 5]. Moreover, several studies revealed that fracture risk is increased in primary hyperparathyroidism patients [6, 7]. Numerous factors affect BMD in women; however, the relationships between glucose metabolism and BMD in patients with primary hyperparathyroidism remain unclear.
BMD is significantly increased at the lumbar spine and femoral neck in patients with type 2 diabetes mellitus [8, 9], suggesting that glucose metabolism positively affects BMD; however, the effects of fasting plasma glucose (FPG) levels on bone within the physiological ranges are unknown. As for the relationships between glucose metabolism and primary hyperparathyroidism, several groups have reported that the prevalence of impaired glucose metabolism was significantly higher in primary hyperparathyroidism patients than in non-primary hyperparathyroidism patients [10–13]; however, how glucose levels affect bone in patients with primary hyperparathyroidism is unknown. Therefore we hypothesized that FPG within the physiological ranges might be positively related to bone metabolism in patients with primary hyperparathyroidism. Decreased BMD is one of the most important clinical parameters for the indication of surgery and the follow up of patients with primary hyperparathyroidism. Since BMD measurements are not available in numerous clinics, the search for other parameters, which are frequently and easily measurable, may be useful for the clinical assessment of patients with primary hyperparathyroidism. Moreover, how muscle and fat mass affect bone metabolism in patients with primary hyperparathyroidism is unknown.
The present study was therefore performed to examine the relationships between FPG and the indices related to bone metabolism, including BMD, in 93 postmenopausal women with primary hyperparathyroidism.
Materials and methods
Subjects
Ninety-three postmenopausal women, diagnosed with primary hyperparathyroidism, participated in this study. All subjects were free of drugs known to influence bone metabolism until the time of the present study. In all primary hyperparathyroidism enrolled in the present study, abnormal parathyroid gland swelling was successfully identified by at least two imaging techniques among ultrasonography, computed tomography, magnetic resonance imaging, or technetium sestamibi scintigraphy; and the biochemical data were compatible with primary hyperparathyroidism. Moreover, familial hypocalciuric hypercalcemia was excluded, based on a low calcium (Ca)/creatinine (Cr) clearance ratio by 24-hour urine collection. No patients had complications that might cause changes in bone metabolism. None of the patients suffered from any disease causing osteoporosis.
The study was approved by the ethics review board of Kobe University Hospital. All subjects agreed to participate in the study and gave informed consent.
Biochemical measurements
Serum and urinary chemistry determinations were performed by standard automated techniques. Serum chemistry was performed in daily routine assays. Urine was collected as second void morning urine except for measurement of the Ca/Cr clearance ratio. Serum concentrations of intact PTH were measured by immunoradiometric assay (Allegro Intact PTH IRMA kit; Nichols Institute Diagnostics, San Juan Capistrano, Ca; normal range, 10–65 pg/ml). Serum levels of bone-type alkaline phosphatase (normal range: 7.9–29.0 U/L) and osteocalcin (normal range: 2.5–13 ng/ml) as well as urinary levels of deoxypyridinoline (normal range: 2.8–7.6 nmol/mmol.Cr) and type I collagen cross-linked N-telopeptides (normal range: 9.3–54.3 nmolBCE/mmolCr) were measured as previously described [3, 14, 15]. Immunoreactive insulin was measured as previously described [16]. Immunoreactive insulin levels were available for only 44 patients among the subjects.
BMD measurements by dual-energy X-ray absorptiometry
BMD values were measured by dual-energy X-ray absorptiometry using QDR-2000 (Hologic Inc., Waltham, MA) at the lumbar spine (L2–4), femoral neck, and distal one third of the radius. BMD was automatically calculated from the bone area (cm2) and bone mineral content (g), and expressed absolutely in g/cm2. The coefficients of variation (precision) of measurements at the lumbar spine, femoral neck and radius were 0.9, 1.7 and 1.9%, respectively. The Z-score is the number of standard deviation (SD) by which a given measurement differs from the mean for a sex-, age-, and race-matched reference population. The T-score is the number of SD by which a given measurement differs from the mean for a normal young adult reference population. The coefficient of variation was obtained in vitro using a ‘phantom’ with at least four measurements for the same subject. Normative data were obtained from a population-based database of the Japanese Society of Bone and Mineral Research, 1996.
Measurement of body composition
Dual-energy X-ray absorptiometry enables us to analyze body composition in terms of lean body mass and fat mass easily and precisely [17]. Lean body mass and fat mass were measured by dual-energy X-ray absorptiometry (QDR-2000 Hologic Inc., Boston, MA, USA), using whole-body absorptiometry software and expressed in kg. Percentage fat mass (% fat) was calculated by dividing the absolute value of body composition by total body mass. The same operator tested all the women during the study to eliminate operator discrepancies. A strong correlation between body weight and total body mass measured by dual-energy X-ray absorptiometry (r=0.98) was obtained in a preliminary study. Coefficients of variation of measurements of lean body mass and fat mass were 1.0 and 2.0 %, respectively. The coefficient of variation was obtained in vitro using a ‘phantom’.
Statistical analysis
All data are expressed as the mean ± SD for each index. Statistical analyses were performed using StatView IV (Abacus Concepts, Inc., Berkley, CA, USA). Simple or multiple regression analyses were performed to assess the relationships among several parameters, and Pearson's correlation coefficients were calculated. Values of p < 0.05 were considered significant.
Results
Background data
Background data of postmenopausal women with primary hyperparathyroidism are shown in Table 1. Their age was 64.4± 8.9 years. Serum Ca levels were 11.4 ± 1.4 mg/dl. Serum levels of PTH and non-specific alkaline phosphatase were higher, compatible with the biochemical data of primary hyperparathyroidism. Serum Cr and Cr clearance levels were 0.66 ± 0.22 mg/dl and 83.2 ± 31.6 ml/min, respectively, although the patients with Cr clearance levels less than 40 ml/min were not included in this study. BMD (Z-score) at all sites was low. As for bone metabolic indices, serum levels of osteocalcin and bone-type alkaline phosphatase, and urinary levels of type I collagen crosslinked N-telopeptides and deoxypyridinoline were 19.7 ± 15.0 ng/dl, 61.8 ± 44.2 U/L, 126 ± 112 nmol/mmolCr, 14.3 ± 10.0 nmolBCE/mmolCr, respectively. FPG levels were 94.0 ± 17.4 mg/dl and more than 110 mg/dl in only 13 patients. Mean immunoreactive insulin levels were 9.6 ± 8.2 U/ml.
Table 1.
Background data in primary hyperparathyroidism women
| Primary hyperparahyroidism | |
|---|---|
| Age (years) | 64.4 ± 8.9 |
| Height (cm) | 151.4 ± 6.1 |
| Body weight (kg) | 52.3 ± 8.7 |
| BMI (kg/m2) | 22.8 ± 3.7 |
| Ca (mg/dl) | 11.4 ± 1.4 |
| ALP (IU/L) | 421 ± 223 |
| PTH (pg/ml) | 195 ± 176 |
| FPG (mg/dl) | 94.0 ± 17.4 |
| L-BMD T score | −2.185 ± 1.518 |
| Z score | −0.719 ± 1.127 |
| FN-BMD T score | −1.992 ± 0.963 |
| Z score | −0.565 ± 0.975 |
| Rad1/3-BMD T score | −4.099 ± 1.943 |
| Z score | −1.203 ± 1.826 |
ALP, alkaline phosphatase;L, lumbar; FN, femoral neck; Rad1/3, distal radius
Relationships between FPG or immunoreactive insulin and various parameters in postmenopausal primary hyperparathyroidism women Correlations between FPG or immunoreactive insulin and various indices were examined in postmenopausal primary hyperparathyroidism women. As shown in Table 2, body weight and BMI were significantly and positively related to both FPG and immunoreactive insulin, although age, height and serum albumin levels showed no relation. As for the body composition index, fat mass as well as % fat, but not lean body mass, were positively related to FPG, although only % fat was significantly related to immunoreactive insulin levels.
Table 2.
Correlations between FPG or immunoreactive insulin and various indices in postmenopausal primary hyperparathyroidism women
| FPG | IRI | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.112 | 0.2858 | −0.060 | 0.7025 |
| Height | −0.011 | 0.9177 | 0.172 | 0.2775 |
| Body weight | 0.252** | 0.0177 | 0.437* | 0.0035 |
| BMI | 0.258** | 0.0151 | 0.378** | 0.0129 |
| Albumin | 0.020 | 0.8516 | −0.148 | 0.3399 |
| Lean Body Mass | 0.084 | 0.5213 | −0.069 | 0.7315 |
| Fat Mass | 0.282** | 0.0274 | 0.253 | 0.1953 |
| % Fat | 0.279** | 0.0293 | 0.441** | 0.0180 |
IRI, immunoreactive insulin;
p<0.01,
p<0.05
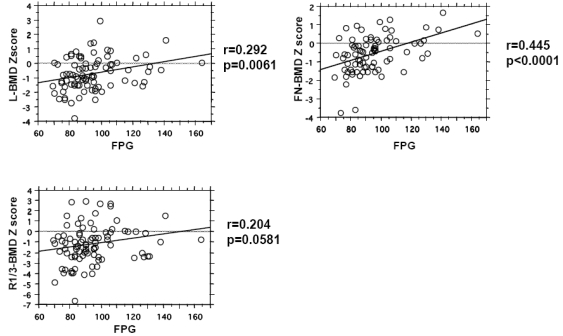
Next, we examined the relationships between FPG or immunoreactive insulin and bone metabolism parameters in these patients. Serum levels of calcium, PTH and 1, 25-dihydroxyvitamin D3 as well as any bone metabolic indices were not related to FPG and immunoreactive insulin levels in postmenopausal primary hyperparathyroidism women (Table 3). As for BMD, FPG levels were positively related to Z scores of BMD at the lumbar spine and femoral neck (Figure 1), although they were not significantly related to the Z-score of BMD at the radius. On the other hand, immunoreactive insulin levels were not significantly related to BMD parameters at any sites (Table 3).
Table 3.
Correlations between FPG or immuoreactive insulin and various bone metabolic indices in postmenopausal primary hyperparathyroidism women
| FPG | IRI | |||
|---|---|---|---|---|
| r | p | r | p | |
| Ca | 0.103 | 0.3295 | 0.120 | 0.4383 |
| PTH | −0.163 | 0.1217 | −0.262 | 0.0854 |
| 1,25-dihydroxyvitaminD3 | −0.150 | 0.2233 | −0.195 | 0.2435 |
| BAP | 0.014 | 0.9224 | −0.105 | 0.5711 |
| OCN | −0.223 | 0.0654 | −0.112 | 0.5013 |
| NTX | −0.178 | 0.1626 | −0.235 | 0.1815 |
| DPD | −0.231 | 0.0995 | −0.100 | 0.5942 |
| L-BMD Z score | 0.292* | 0.0061 | 0.183 | 0.2607 |
| FN-BMD Z score | 0.445* | <0.0001 | 0.292 | 0.0637 |
| R1/3-BMD Z score | 0.204 | 0.0581 | 0.0075 | 0.6474 |
BAP, bone-type alkaline phosphatase; OCN, osteocalcin; NTX, type I collagen cross-linked N-telopeptides; DPD, deoxypyridinoline; L, lumbar; FN, femoral neck; Rad1/3, distal radius; IRI, immunoreactive insulin;
p<0.01,
p<0.05
Figure 1.
Relationships between FPG and BMD (Z score) in postmenopausal primary hyperparathyroidism women. Simple regression analysis was performed, and Pearson's correlation coefficients (r) were calculated. P values <0.05 were considered significant. L, lumbar; FN, femoral neck; Rad1/3, distal radius;
Relationships between BMD and various indices in postmenopausal primary hyperparathyroidism women
Since BMD at the lumbar spine and femoral neck was significantly related to FPG, we examined the relationships between BMD (Z scores) and various indices in postmenopausal primary hyperparathyroidism women. As shown in Table 4, BMD (Z score) at both sites was significantly related to body weight, BMI, serum levels of PTH, osteocalcin, urinary type I collagen cross-linked Ntelopeptides levels, fat mass and % fat. We therefore examined whether these factors would affect the relationships between FPG and BMD at the lumbar spine or femoral neck by using multiple regression analyses, as shown in Table 5. FPG was significantly related to BMD (Z score) at the lumbar spine, when body weight, BMI, PTH, type I collagen cross-linked N-telopeptides or deoxypyridinoline were considered; however, these relationships were not significant when fat mass or % fat was considered. These findings indicated that FPG was related to lumbar BMD associated with fat. The relationships between FPG and BMD (Z score) at the femoral neck were independent of body weight, BMI, PTH, bone metabolic indices, fat mass or % fat. In 44 subjects in whom immunoreactive insulin levels were measured, FPG levels were significantly related to BMD (Z score) at the lumbar spine and femoral neck when immunoreactive insulin levels were considered (lumbar spine: r=0.332, p=0.0406; femoral neck: r=0.548, p=0.0002).
Table 4.
Correlations between BMD and various indices in postmenopausal primary hyperparathyroidism women
| L-BMD Z score | FN-BMD Z score | |||
|---|---|---|---|---|
| r | p | r | p | |
| Body Weight | 0.411* | <0.0001 | 0.424* | <0.0001 |
| BMI | 0.381* | 0.0003 | 0.366* | 0.0007 |
| PTH | −0.380* | 0.0003 | −0.366* | 0.0007 |
| OCN | −0.381* | 0.0017 | −0.254** | 0.0416 |
| NTX | −0.440* | 0.0005 | −0.284** | 0.0291 |
| DPD | −0.529* | <0.0001 | −0.248 | 0.0827 |
| Fat Mass | 0.305** | 0.0174 | 0.423* | 0.0006 |
| % Fat | 0.294** | 0.0220 | 0.378* | 0.0024 |
OCN, osteocalcin; NTX, type I collagen cross-linked N-telopeptides;DPD, deoxypyridinoline; L, lumbar; FN, femoral neck;
p<0.01,
p<0.05
Table 5.
Correlations between FPG and BMD in multiple regression analysis
| FPG vs L-BMD (Z score) | FPG vs FN-BMD (Z score) | |||
|---|---|---|---|---|
| r | p | r | p | |
| Body Weight | 0.205** | 0.0469 | 0.368* | 0.0003 |
| BMI | 0.208** | 0.0481 | 0.380* | 0.0003 |
| PTH | 0.237** | 0.0204 | 0.409* | <0.0001 |
| OCN | 0.234 | 0.0525 | 0.397* | 0.0011 |
| NTX | 0.274** | 0.0239 | 0.380* | 0.0026 |
| DPD | 0.311** | 0.0126 | 0.385* | 0.0060 |
| Fat Massx | 0.134 | 0.3084 | 0.265** | 0.0299 |
| % Fat | 0.138 | 0.2952 | 0.279** | 0.0247 |
Multiple regression analysis was performed with BMD (Z score) at each site as dependent variables, and FPG and each parameter as independent variables. OCN, osteocalcin; NTX, type I collagen cross-linked N telopeptides; DPD, deoxypyridinoline; L, lumbar; FN, femoral neck;
p<0.01,
p<0.05
Discussion
In the present study, FPG was positively related to Z-scores of BMD at the lumbar spine and femoral neck in postmenopausal primary hyperparathyroidism women, indicating that an increase in FPG is protective against a BMD decrease by primary hyperparathyroidism. How FPG is related to bone metabolism in primary hyperparathyroidism is unknown. Our recent study revealed that FPG is positively related to BMD increase at the lumbar spine by bisphosphonate treatment in patients with postmenopausal osteoporosis [18], suggesting that FPG changes in the physiological range are related to bone metabolism in osteoporotic patients. Since only a small number of patients were considered to be diabetes mellitus in the present study, the influence of FPG does not seem to be due to high glucose levels or impaired glucose intolerance.
Fat mass and lean body mass affects BMD [19–21]. Our previous study indicated that lean body mass is a more important determinant of BMD than fat mass at any site, except the femoral neck, in postmenopausal Japanese women [22]. Another report agreed with the importance of lean body mass in the protection against postmenopausal osteoporosis [19], although several studies suggested the importance of both lean body mass and fat mass [20, 21]. Moreover, BMI was a determinant of BMD increase in the lumbar spine and femoral neck in patients with type 2 diabetes mellitus [8]. In the present study, fat mass as well as % fat were positively related to BMD (Z score) at the lumbar spine and femoral neck in postmenopausal primary hyperparathyroidism women. Moreover, FPG was positively related to fat mass and % fat, but not lean body mass in these patients. In multiple logistic analysis, FPG was not significantly related to BMD (Z score) at the lumbar spine when fat mass or % fat was considered, indicating that the relationships between FPG and BMD at the lumbar spine are associated with fat mass. FPG was significantly related to BMD (Z-score) at the lumbar spine and femoral neck when lean body mass was considered in multiple logistic analysis (data not shown). These findings suggest that fat tissues are more important than muscle tissues in the relationships between FPG and BMD in postmenopausal pHPT women. Some factors from fat tissues might simultaneously affect FPG and BMD to suppress BMD decrease by PTH excess in primary hyperparathyroidism patients. Insulin is the most important hormone, affecting FPG, fat and BMD. In the present study, immunoreactive insulin levels were positively related to body weight, BMI and % fat, although they were not significantly related to BMD (Z score) at any site. The extent of the relation to fat or BMD parameters seemed to be different between FPG and immunoreactive insulin. Moreover, multiple regression analysis indicated that FPG was related to BMD at the lumbar spine and femoral neck independently of immunoreactive insulin. These findings suggest that immunoreactive insulin cannot explain the relationships between FPG and BMD at the lumbar spine and femoral neck in postmenopausal primary hyperparathyroidism women in the present study. Other systemic factors might simultaneously affect FPG, fat mass and bone mass in primary hyperparathyroidism patients.
The present study has some limitations. First, the sample size was not large enough to reach definite conclusions. Second, data of 25-hydroxyvitamin D were not obtained for the assessment of vitamin D status in the present study. Thus, the differences in vitamin D status might affect the severity of disease, bone turnover and BMD in primary hyperparathyroidism patients, because our previous study with 30 postmenopausal women with primary hyperparathyroidism suggested that the vitamin D status affects BMD [23].
In normal subjects or primary osteoporotic patients, whether FPG levels are related to BMD is unknown. Although BMD increase has been reported in patients with type 2 diabetes mellitus [8,9], FPG levels themselves were not related to BMD in patients with type 2 diabetes mellitus. Therefore, the present study is the first report, to our knowledge, describing that FPG levels were positively related to BMD in secondary osteoporosis.
In conclusion, the present study first indicated that FPG levels were positively related to BMD at the lumbar spine and femoral neck in postmenopausal women with primary hyperparathyroidism. Some systemic factors, which simultaneously affect FPG, fat mass and bone mass, might be responsible for these mechanisms.
References
- 1.Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- 2.Kaji H, Yamauchi M, Chihara K, Sugimoto T. The threshold of bone mineral density for vertebral fractures in female patients with primary hyperparathyroidism. Eur J Endocrinol. 2005;153:373–378. doi: 10.1530/eje.1.01985. [DOI] [PubMed] [Google Scholar]
- 3.Kaji H, Nomura R, Yamauchi M, Chihara K, Sugimoto T. The usefulness of bone metabolic indices for the prediction of changes in bone mineral density after parathyroidectomy in patients with primary hyperparathyroidism. Horm Metab Res. 2006;38:411–416. doi: 10.1055/s-2006-944533. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Kaji H, Iu MF, Nomura R, Sowa H, Yamauchi M, Tsukamoto T, Sugimoto T, Chihara K. Effects of an excess and a deficiency of endogenous parathyroid hormone on volumetric bone mineral density and bone geometry determined by peripheral quantitative computed tomography in female subjects. J Clin Endocrinol Metab. 2003;88:4655–4658. doi: 10.1210/jc.2003-030470. [DOI] [PubMed] [Google Scholar]
- 5.Kaji H, Yamauchi M, Nomura R, Sugimoto T. Improved peripheral cortical bone geometry after surgical treatment of primary hyperparathyroidism in postmenopausal women. J Clin Endocrinol Metab. 2008;93:3045–3050. doi: 10.1210/jc.2007-2480. [DOI] [PubMed] [Google Scholar]
- 6.Melton LJ, III, Atkinson EJ, O'Fallon WM, Heath H., III Risk of age-related fractures in patients with primary hyperparathyroidism. Arch Intern Med. 1992;152:2269–2273. [PubMed] [Google Scholar]
- 7.Khosla S, Melton LJ, III, Wermers RA, Crowson CS, O'Fallon WM, Riggs B. Primary hyperparathyroidism and risk of fracture: a population-based study. J Bone Miner Res. 1999;14:1700–1707. doi: 10.1359/jbmr.1999.14.10.1700. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 9.de Liefde, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 10.Richards ML, Thompson NW. Diabetes mellitus with hyperparathyroidism: another indication for parathyroidectomy? Surgery. 1999;126:1160–1166. doi: 10.1067/msy.2099.101436. [DOI] [PubMed] [Google Scholar]
- 11.Taylor WH, Khaleeli AA. Coincident diabetes mellitus and primary hyperparathyroidism. Diabet Metab Res Rev. 2001;17:175–180. doi: 10.1002/dmrr.199. [DOI] [PubMed] [Google Scholar]
- 12.Procopio M, Magro G, Cesario F, Piovesan A, Pia A, Molineri N, Borretta G. The oral glucose tolerance test reveals a high frequency of both impaired glucose tolerance and undiagnosed Type 2 diabetes mellitus in primary hyperparathyroidism. Diabet Med. 2002;19:958–961. doi: 10.1046/j.1464-5491.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- 13.Khaleeli AA, Johnson JN, Taylor WH. Prevalence of glucose intolerance in primary hyperparathyroidism and the benefit of parathyroidectomy. Diabet Metab Res Rev. 2007;23:43–48. doi: 10.1002/dmrr.637. [DOI] [PubMed] [Google Scholar]
- 14.Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7:1251–1258. doi: 10.1002/jbmr.5650071119. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto T, Kaji H, Nakaoka D, Yamauchi M, Yano S, Sugishita T, Baylink DJ, Mohan S, Chihara K. Effect of low-dose of recombinant human growth hormone on bone metabolism in elderly women with osteoporosis. Eur J Endocrinol. 2002;147:339–348. doi: 10.1530/eje.0.1470339. [DOI] [PubMed] [Google Scholar]
- 16.Fukuyama K, Ohara T, Hirota Y, Maeda K, Kuno S, Zenibayashi M, Teranishi T, Kouyama K, Maeda E, Sakamoto N, Kasuga M. Association of the -112A>c polymorphism of the uncoupling protein 1 gene with insulin resistance in japanese individuals with type 2 diabetes. Biochem Biophys Res Commun. 2006;339:1212–1216. doi: 10.1016/j.bbrc.2005.11.140. [DOI] [PubMed] [Google Scholar]
- 17.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual energy X-ray absorptiometry for total-body and regional bone mineral and soft tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 18.Kaji H, Hisa I, Inoue Y, Naito J, Sugimoto T, Kasuga M. Analysis of factors affecting an increase in bone mineral density at lumbar spine by bisphosphonates in postmenopausal osteoporosis. J Bone Miner Metab in press. doi: 10.1007/s00774-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 19.Bevier WC, Wiswell RA, Pyka G, Kozak KC, Newhall KM, Marcus R. Relationship of body composition, muscle strength and aerobic capacity to bone mineral density in older men and women. J Bone Miner Res. 1998;4:421–432. doi: 10.1002/jbmr.5650040318. [DOI] [PubMed] [Google Scholar]
- 20.Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, Lim TM, Cundy TF. Determinants of total body and regional bone mineral density in normal postmenopausal women: a key role for fat mass. J Clin Endocrinol Metab. 1992;75:45–51. doi: 10.1210/jcem.75.1.1619030. [DOI] [PubMed] [Google Scholar]
- 21.Khosla S, Atkinsen EJ, Riggs BL, Melton LJ., III Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857–863. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 22.Nakaoka D, Sugimoto T, Kaji H, Kanzawa M, Yano S, Yamauchi M, Sugishita T, Chihara K. Determinants of bone mineral density and spinal fracture risk in postmenopausal Japanese women. Osteoporos Int. 2001;12:548–554. doi: 10.1007/s001980170075. [DOI] [PubMed] [Google Scholar]
- 23.Inoue Y, Kaji H, Hisa I, Tobimatsu T, Naito J, Iu MF, Sugimoto T, Chihara K. Vitamin D status affects osteopenia in postmenopausal patients with primary hyperparathyroidism. Endocr J. 2008;55:57–65. doi: 10.1507/endocrj.k07-102. [DOI] [PubMed] [Google Scholar]