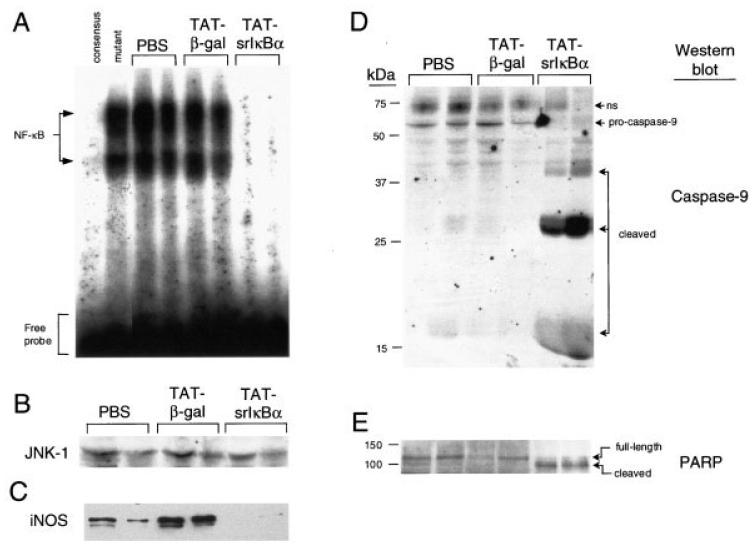
Figure 2.
Reduced NF-κB activity, but elevated caspase activation, in inflammatory neutrophils from Tat-srIκBα–treated animals. A, DNA-binding activity of NF-κB in cell lysates of infiltrating neutrophils obtained from 6-hour pleural exudates following systemic administration of Tat fusion proteins or phosphate buffered saline (PBS) vehicle. Results from 2 animals from each treatment group are shown. Protein/oligonucleotide complexes specific for NF-κB were determined by competition with unlabeled NF-κB consensus or mutant oligonucleotides, using inflammatory cell lysates from PBS-treated animals. Protein concentrations in cell lysates were quantified with the bicinchoninic acid kit. B, Western blotting with anti–JNK-1 antibodies (loading control). Similar aliquots from these lysates were probed with antibodies to C, inducible nitric oxide synthase (iNOS) to determine the expression of NF-κB-regulated genes, and with antibodies to D, caspase-9 and E, poly(ADP-ribose) polymerase (PARP) to detect caspase-mediated cleavage of protein substrates. Full-length proteins and their cleaved products are indicated; ns = nonspecific.