Abstract
Temporal advancement of resource availability by warming in seasonal environments can reduce reproductive success of vertebrates if their own reproductive phenology does not also advance with warming. Indirect evidence from large-scale analyses suggests, however, that migratory vertebrates might compensate for this by tracking phenological variation across landscapes. Results from our two-year warming experiment combined with seven years of observations of plant phenology and offspring production by caribou (Rangifer tarandus) in Greenland, however, contradict evidence from large-scale analyses. At spatial scales relevant to the foraging horizon of individual herbivores, spatial variability in plant phenology was reduced—not increased—by both experimental and observed warming. Concurrently, offspring production by female caribou declined with reductions in spatial variability in plant phenology. By highlighting the spatial dimension of trophic mismatch, these results reveal heretofore unexpected adverse consequences of climatic warming for herbivore population ecology.
Keywords: caribou, climate change, global warming, life history, plant phenology, population dynamics
1. Introduction
The heterogeneous distribution of resources in time and space has long been recognized as an important factor in population dynamics (Roughgarden 1974; Levin 1976). Indeed, in many systems, successful survival and reproduction by individuals depends upon the strategies employed in coping with temporal and spatial variability in resources (Giesel 1976; Wiens 1976). In seasonal northern environments, migration confers a selective advantage related to maximization and prolongation of the intake of high-quality forage during the season of highest reproductive costs (Klein 1970; White 1983; Lundberg 1988; Kingsolver et al. 2002). Influences of climatic warming on the dynamics of resource availability in time and space may, therefore, pose important consequences for the reproductive success of migratory animals.
Primarily for this reason, the role of temporal shifts in resource availability associated with warming has received considerable attention in development of the trophic mismatch concept (Visser et al. 1998) and documentation of its consequences for reproductive success in migratory birds. For instance, flycatchers (Ficedula sp.) in Europe appear to time their migration to breeding areas on the basis of light cues, whereas the timing of emergence of their forage resources is cued by local temperatures on the breeding grounds; as springtime temperatures increase, resources emerge earlier and there is a consequent gap between resource availability and resource demand by breeding pairs (Both et al. 2004; Visser et al. 2004; Visser & Both 2005).
On the contrary, far less attention has been devoted to the spatial dimension of temporal shifts in resource availability associated with climatic warming. For large-bodied migratory herbivores, this dimension may be particularly important (Mårell et al. 2002). In the most seasonal environments, large herbivores display a migratory strategy that, whether it evolved in response to predation pressure or not, enables them to follow the spatial front of emerging vegetation, prolonging the period of intake of the most digestible, highly nutritious plants (Klein 1970; Skogland 1989; Gunn & Skogland 1997; Ferguson & Elkie 2004). Red deer (Cervus elaphus), for example, migrate upslope along elevational gradients in spring as green-up advances (Albon & Langvatn 1992), and the timing and synchrony of their offspring production is closely cued to the onset and progression of the plant growing season (Loe et al. 2005). Caribou (Rangifer tarandus) also time their spring migrations to coincide with the emergence of nutritious, highly digestible plant tissues to support the high costs of lactation (Klein 1970; White 1983). Indeed, among populations, variation in timing of parturition by caribou corresponds with variation in the onset of plant growth on the calving grounds of those populations (Skogland 1989; Post et al. 2003). In the context of climate change, however, a crucial consideration is whether advances in phenology at lower trophic levels related to climatic warming will be matched by the species at higher trophic levels that depend on them for successful reproduction (Both & Visser 2005). At least one multi-annual study of a large herbivore population suggests that this may not be the case, as advancement of the onset of the plant growing season in West Greenland has not been matched by advancement of the onset of calving by caribou there, and consequently productivity in that caribou population has declined (Post & Forchhammer 2008).
The manner in which spatial heterogeneity in the timing of plant growth responds to climatic warming is likely, therefore, to be of importance to the reproductive success of herbivores in highly seasonal environments. Considerable effort has been devoted to analysing large-scale spatial patterns of plant phenology in relation to climatic variation and change. Continental-scale analyses of normalized difference vegetation index (NDVI) data spanning thousands of kilometres from north temperate China, for instance, have revealed that large-scale spatial patterns of the onset of plant growth correlate with spatial patterns of variation in spring warming (Chen et al. 2005). Similarly, landscape-scale analyses of data on first flowering dates of plants spanning hundreds of kilometres in Norway revealed earlier onset of flowering following warm winters and springs (Post & Stenseth 1999), and an increase in spatial variability at the same scale following warming (Post & Stenseth 1999; Post 2003). As well, landscape-scale analyses of NDVI data from Norway have shown an increase in spatial variability in the timing of the onset of plant growth following warm winters, presumably owing to accentuation of terrain–snowmelt interactions (Pettorelli et al. 2005a,b).
Such analyses suggest that warming increases the spatial variability of timing of phenological events in plants. However, the results described above were based on analyses of coarsely resolved, landscape-scale, time-series data. In contrast to the patterns observed at the scale of the landscape, warming might not, at the local scale (i.e. metres to hundreds of metres), increase spatial heterogeneity of phenological events, but instead could reduce it. This might be the case for at least two reasons. First, at such small spatial scales there is less pronounced variation in aspect, slope and elevation, all of which interact with temperature and precipitation at the landscape scale; second, alleviation of temperature constraints on the timing of life-history events by warming at the local scale might be expected to hasten phenological development among individuals co-occurring at that scale (Post et al. 2008). Thus, our objectives were to conduct a plot scale warming experiment designed to determine whether the relationships between warming and spatial dynamics of plant phenology observed at scales of hundreds to thousands of kilometres were mirrored by patterns at the much smaller scale of metres to hundreds of metres, at which individual herbivores forage; to determine whether the results of our experiment accorded with multi-annual observational data at the same spatial scales; and to examine the consequences of relationships between observed warming and spatial dynamics of plant phenology for herbivore productivity.
2. Material and methods
(a) Plant phenological response to experimental warming
We conducted our warming experiment in a low-arctic plant community near Kangerlussuaq, West Greenland (67°6.8′ N, 50°20′ W). This area comprises non-carbonate mountain complexes with the dominant vegetation type characterized as low-shrub tundra (CAVM Team 2003; see also Post et al. 2003), and is inhabited by caribou and muskoxen (Ovibos moschatus). In June 2002, we erected six 800 m2 exclosures as part of a large-scale experiment designed to quantify the influences of herbivory and warming on primary productivity and plant community dynamics. The exclosures were circular and constructed of steel T-posts and woven wire fence measuring 120 cm high. Our warming experiment was conducted inside three of these exclosures. The mean (±1 s.e.) distance among our exclosures was 621.4±151 m.
In May 2003, before the onset of plant growth, we erected four open-topped passive warming chambers (OTCs) inside each of three of the exclosures. The OTCs were constructed of UV-neutral glazing material that was 0.10 cm thick (Sun-Lite HP, Solar Components Corporation). We followed the protocol of the International Tundra Experiment in designing our OTCs, which were cone shaped, with a 60° side angle, approximately 150 cm in diameter at the base, 1.77 m2 in area and approximately 40 cm high (Marion et al. 1997). The OTCs elevate near-surface temperatures but minimize unwanted side effects, such as interfering with gas exchange and evaporation and precipitation (Marion et al. 1997). Use of the term ‘treatment plot’ hereafter refers to experimental plots warmed with OTCs. Adjacent to each treatment plot, but at least 3 m away, we also demarcated a control plot of the same dimensions; plots of both types were equipped with a surface thermometer and hygrometer. All plots were positioned at a minimum of 2 m away from the inside of exclosure fencing to minimize edge effects and at least 3 m away from the nearest treatment or control plot.
At the time of establishment of our treatment and control plots, we recorded all plant species present and their phenological states. We revisited the site on 3 June 2003 and remained onsite until termination of the experiment on 19 June 2003. All plots were visited on a daily or bi-daily basis, when we recorded the phenological stages of all species present in each plot, as well as surface temperature and humidity. On 19 June 2003 all OTCs were taken down. We revisited the site 18 May 2004, when all OTCs, thermometers and hygrometers were replaced on the original plots. At the time of re-establishment of the warming experiment in 2004, we recorded all species present and their phenological states. In 2004, observations recommenced on 4 June and terminated on 20 June, following the exact procedures used in 2003. In 2003, temperature and humidity were recorded on all plots for a total of 11 days; in 2004 they were monitored for 14 days. Mean daily temperature was calculated as the average of the daily maximum and minimum temperatures, and was compared between treatment and control plots using ANOVA with treatment effect as a fixed factor, and day of year as a continuous random covariate.
(b) Timing of phenological events and intervals between them
As a precursor to investigating influences of warming on spatial dynamics of plant phenology, first it was necessary to quantify influences of warming on timing of phenological events. We compared the timing of phenological events, including emergence in forbs and leaf opening (LO) in deciduous shrubs, flower set (FS), blooming (B) and fruit set (FR), within each species monitored, between treatment and control plots. We used a univariate ANOVA with treatment effect and ‘year’ as fixed factors, and the June date of first observation of each event for each species observed on each plot as the response; the ANOVA included ‘site’ and ‘plot’ as random covariates, with plot nested within site.
We investigated the influence of warming on the progression of phenology by comparing the mean interval between successive phenological events on treatment and control plots. We calculated the number of days between first observation of successive events for each species within each plot and compared means for each phenological interval within each species among treatment and control plots in a univariate ANOVA. In contrast to our previous analysis (Post et al. 2008), we did not attempt to track phenology in individual plants. Although we did in fact record successive events observed on each plot for each species, we used the intervals between the first date for each event within species among all plots in this analysis. As stated, our intent was to compare the spatial heterogeneity of plant phenology between treatment and control plots. Because previous results from this experiment revealed that warming hastened phenological sequences in several species (Post et al. 2008), we expected warming to reduce spatial variability in plant phenology among plots. The relevance of this analysis to herbivore foraging ecology is that it quantifies the influence of warming on the progression of the plant growing season at local and intermediate spatial scales. This presumably reflects the resource state in space and time that herbivores might be exposed to in a warmer climate.
In all cases, ANOVAs included year as a fixed factor to test for between-year variation. A significant between-year effect was detected only for emergence in Stellaria longipes, and for blooming in Cerastium alpinum and Carex supina. For events for which there was no significant year effect, data from both years were pooled. For the events for which we detected a significant effect of year, means are reported after accounting for this effect. Results for the timing of phenological events are reported as the June date of the event.
The numbers of treatment and control plots on which we observed multiple phenological events within each species limited our analyses of phenological dynamics. The earliest phenological event observed in most cases was emergence in forbs and its analogue in deciduous shrubs, LO; this was observed for C. alpinum, Draba sp., C. supina, S. longipes and Salix glauca. Emergence and LO were also observed, but not on a sufficient number of plots for analysis, in Potentilla palustris and Betula nana. Following emergence, FS was observed for C. alpinum, S. longipes, S. glauca and B. nana. Blooming was observed in C. alpinum, Draba sp., C. supina, S. glauca, B. nana and P. palustris. Finally, FR was observed in B. nana, Draba sp. and C. supina.
(c) Spatial variability in phenological events
We examined spatial variability in phenological progression at two scales: an intermediate scale, with comparisons among plots over distances of hundreds of metres, and a local scale, with comparisons among plants within plots, over distances of 1 m or less. To quantify the influence of warming on spatial variability of plant phenology at the intermediate spatial scale, we used F-tests of variances around the means of each event observed for each species among all plots of each type (treatment or control). In quantifying the influence of warming on local-scale spatial dynamics of plant phenology, we calculated the coefficient of variation (CV) of the dates of all events observed within each plot and then used an ANOVA to test for differences between treatment and control plots. The ANOVA included plot and site as random effects, neither of which was significant, and year as a fixed factor.
(d) Observed spatio-temporal variation in plant phenology and herbivore reproduction
We monitored emergence of plant species on permanent plots within the calving area of caribou in our study site in 1993 and from 2002 to 2007 as described in detail previously (Post & Klein 1999; Post et al. 2003, 2008). We quantified the progression of the plant growing season using the nonlinear regression model,
| (2.1) |
in which Y is the daily proportion of the final number of species emergent and X is the Julian date. In this model, the intercept a quantifies the date at which species begin emerging, and the slope b quantifies the rate of species emergence. We used estimates of a and b from (2.1) to estimate the date of onset of plant growth in each year as the date at which 5% of species had emerged. Spatial variation in the progression of the plant growing season was quantified in two ways: first, as the standard error of the estimate of b from (2.1) with data from all of our phenology plots pooled in each year; second, as the standard error of the mean of b estimated for each plot individually each year. These approaches gave similar results, but we report results using both methods rather than choosing one set of results post priori.
Previously, we reported that offspring production by caribou in our study site was inversely related to an index of trophic mismatch quantified as the per cent of forage species emergent at the midpoint of the calving season each year (Post & Forchhammer 2008). This index of trophic mismatch quantifies the temporal state of the plant growing season, and hence resource availability, in relation to the timing of offspring production and hence peak resource demands for offspring provisioning. Here, we investigated the consequences for offspring production by caribou of spatial variation in timing and progression of the plant growing season. We focused on offspring production because declines in summer calf-to-cow ratios and calf proportions have been associated with declines in other caribou populations in Alaska and Canada (Klein 1968; Ferguson & Mahoney 1991; Collins et al. 2003; Haskell & Ballard 2004; Larter & Nagy 2004), and are presumably an important component of caribou population dynamics. Previously, we reported a positive association between trophic mismatch and early calf mortality in this population (Post & Forchhammer 2008), but did not focus on calf mortality here because that association was not statistically significant.
We performed a multivariate regression analysis of variation in annual offspring production, defined as the peak proportion of calves among the adult female segment of the population each year (Post & Forchhammer 2008), using our index of trophic mismatch and index of spatial variability in the progression of the plant growing season as predictor variables. This model was strictly density independent for two reasons: first, we were constrained by very low sample size (n=7 years of data) and second, our only estimates of population density for each year were our own counts of the total number of caribou observed in the study site each summer. We tested for a relationship between offspring production and caribou density using our estimates of the maximum daily number of caribou observed in the study site each year, and found a negative association suggestive of density-dependent limitation (r=−0.63, p=0.13), but did not pursue a density-dependent model for the reasons stated above.
We began with generalized additive models to test for nonlinear relationships between offspring production and our predictor variables, but those analyses revealed no significant nonlinearity. Hence, we resorted to linear models, of which we report both overall model fit and significance, in addition to partial correlations and significance of each predictor variable. We compared candidate models of offspring production (including trophic mismatch only, spatial variability in phenology only, and both) using corrected Akaike's information criterion (AICc; Sakamoto et al. 1986) scores based on residual sums of squares, sample size and the number of parameters in the model.
3. Results
(a) Experimental warming and timing of events
Mean daily temperature was significantly higher on treatment than on control plots by 2°C in 2003 and 1.4°C in 2004 (Post et al. 2008). Also, daily maximum temperatures were significantly higher on treatment than on control plots in both years (by 3.9°C in 2003 and by 2.6°C in 2004; Post et al. 2008). RH did not differ significantly between treatment and control plots in either year (Post et al. 2008).
Because our phenology results might have been influenced by the number of phenological events observed on treatment and control plots, we used a univariate ANOVA to compare the means of the total number of events observed on each type of plot in each year. The mean (±1 s.e.) number of phenological events observed in 2003 did not differ between treatment (12.4±0.60) and control plots (10.9±0.60; F=3.1, p=0.09). Likewise, the mean number of events observed in 2004 did not differ between treatment (8.92±0.79) and control plots (8.33±0.79).
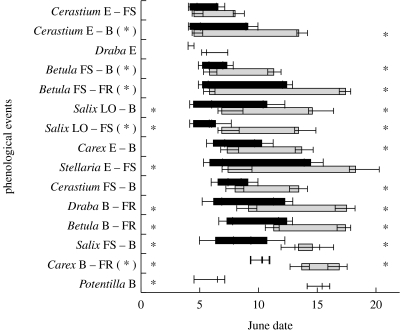
The timing of the earliest phenological events observed, emergence in forbs and LO in deciduous shrubs, occurred significantly earlier on treatment than on control plots for two species: S. longipes and S. glauca (figure 1). Emergence in S. longipes occurred on average on day 5.8 (±0.52) on treatment plots compared with day 7.4 (±0.49) on control plots (F1,42=4.99, p=0.03). LO in S. glauca occurred on average on day 4.5 (±0.32) on treatment plots and on day 6.9 (±0.32) on control plots (F1,45=28.3, p<0.001).
Figure 1.
Timing of plant phenological events on treatment (warmed, black bars) and control (ambient, grey bars) plots at our study site near Kangerlussuaq, West Greenland, in 2003 and 2004. Endpoints of the bars represent the mean (±1 s.e.) dates of the events for each species listed along the l.h.s. of the figure. For each bar, the left endpoint represents the mean date of the first phenological event listed to the l.h.s. of the figure and the right endpoint represents the mean date of the second phenological event listed to the l.h.s. of the figure. The bars represent the mean interval between the pairs of events listed to the l.h.s. of the figure for each species. The events are emergence (E), flower set (FS), blooming (B), fruit set (FR) and leaf opening (LO). The species are listed in §2. Asterisks within the figure denote a significant (p<0.05) difference between treatment and control means for the phenological event represented by the endpoint of the bar. Asterisks outside of the figure and in parentheses denote a significant (p<0.05) difference between treatment and control means for the interval between successive phenological events listed to the l.h.s. of the figure. Events are portrayed in the order in which they occurred on control plots.
FS occurred earlier on treatment than on control plots only for S. glauca (figure 1). For this species, however, sufficient data on timing of FS were available only for 2003, when FS occurred earlier on treatment plots (day 6.33±1.35 versus day 13.4±1.48; F1,9=12.4, p=0.006).
Blooming occurred earlier on treatment than on control plots for several species (figure 1), including C. alpinum (day 10.1±0.53 versus day 14.5±0.90; F1,26=10.0, p=0.004), Draba sp. (day 5.82±0.93 versus day 8.88±0.96; F1,31=5.26, p=0.03), B. nana (day 7.31±0.54 versus day 11.3±0.61; F1,21=23.9, p<0.001), P. palustris (day 6.5±1.99 versus day 15.4±1.26; F1,5=14.3, p=0.01) and C. supina (day 10.1±0.88 versus day 13.4±0.89; F1,26=7.00, p=0.014). As with FS for S. glauca, sufficient data on timing of blooming were available only for 2003, when blooming occurred earlier on treatment (day 9.14±1.1) than on control plots (day 15.8±1.2; F=17.7, p=0.001).
Fruit set occurred earlier on treatment than on control plots for Draba sp., B. nana and C. supina (figure 1). For Draba sp., mean date of FR on treatment plots was day 12.2 (±0.69), but averaged 5 days later on control plots (day 17.5±0.72; F1,23=28.2, p<0.001). Similarly, FR in B. nana occurred on average almost 5 days earlier on treatment (day 12.4±0.48) than on control plots (day 17.4±0.45; F1,19=57.1, p<0.001). For C. supina, sufficient data were available only for 2003, when FR occurred on average 6.5 days earlier on treatment (day 10.3±0.33) than on control plots (day 16.9±0.66; F1,15=51.4, p<0.001).
(b) Phenological intervals among plots
In 5 out of 15 cases, the mean interval from one phenological event to the next successive event among plots was shortened by warming (figure 1). In C. alpinum, progression from emergence to blooming lasted 6.7±0.82 days on treatment plots and 9.8±0.67 days on control plots (F1,26=8.72, p=0.007). In B. nana, progression from FS to bloom lasted 2.8±0.51 days on average on treatment plots compared with 4.8±0.66 days on control plots (F1,14=6.0, p=0.03), whereas the interval between FS and FR lasted 7.1±0.48 days on treatment plots and 11.0±0.59 days on control plots (F1,13=26.3, p<0.001). In S. glauca, the progression from LO to FS lasted 2.2±0.98 days on treatment plots and 6.2±1.1 days on control plots (F1,9=7.73, p=0.02). In C. supina, the transition from blooming to FR lasted 3.7±0.66 days on treatment plots and 6.5±0.78 days on control plots (F1,8=7.58, p=0.03). In only one case was progression delayed by warming: the interval between FS and bloom in S. glauca was shorter on control than on treatment plots, but this difference was not significant (F1,8=0.18, p=0.68).
When arranged in order from earliest to latest occurring events as observed on the control plots, our results suggest that differences between treatment and control means amplified as the growing season progressed (figure 1). We tested for such an effect size using the ratio of control:treatment means for all events observed in an ANOVA with the mean dates of events observed on control plots and ‘start’ or ‘end’ as fixed factors, where these terms indicate whether each event represented the beginning or end of the phenological interval of interest (the endpoints depicted in figure 1). We detected a significant influence of control mean date on effect size (F=8.93, p=0.007), but no influence of start or end (F=1.08, p=0.31). To determine the direction of the influence of control mean date of events on the effect size of our warming treatment, we split the effect size results into groups of initial and terminal events according to the intervals depicted in figure 1. There was a significant relationship between the date of events and effect size for initial events (r=0.65, p<0.02), but no such relationship for terminal events (r=0.06, p>0.50). This suggests that there was a greater response to our warming manipulation among initial events in phenological sequences that occurred later in the growing season. This is also evident in the greater difference between treatment and control means later in the growing season depicted in figure 1.
(c) Spatial variability in plant phenology among and within plots in response to experimental warming
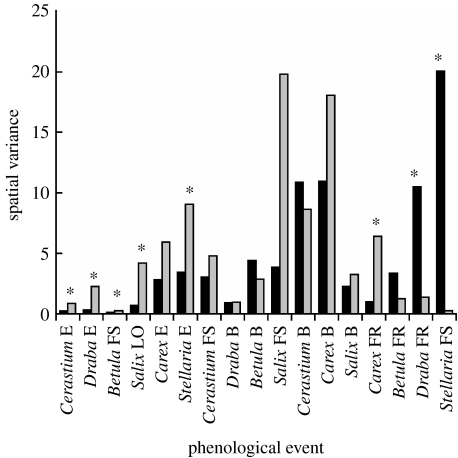
For 12 out of 17 phenological events recorded, warming reduced the spatial variability in the timing of events among plots; however, in only six cases was this reduction statistically significant (p<0.05, F-tests; figure 2). In 5 out of 17 cases, warming increased the spatial variability among plots in the timing of events, but in only two cases was this increase statistically significant (figure 2). Warming significantly reduced the spatial variability among plots in the timing of five of the six earliest events observed, including emergence in C. alpinum, Draba sp. and S. longipes; FS in B. nana, and LO in S. glauca (figure 2). In only one late event, FR in C. supina, did warming reduce spatial variability among plots. By contrast, increases in variability in timing of events among plots in response to warming were isolated to the two latest events observed: FR in Draba sp. and FS in S. longipes (figure 2). For all events that displayed a reduction in spatial variability in response to warming, warming reduced the spatial variability in timing of events among plots by an average of 57.2% (range: 4–86.7%; figure 2). By contrast, the average amount by which warming increased the spatial variability in timing of events among plots was 60.8% (range: 20.6–98.8%; figure 2).
Figure 2.
Spatial variation in phenological events among all treatment (warmed, black bars) and control (ambient, grey bars) plots at our study site near Kangerlussuaq, West Greenland, in 2003 and 2004. Bars represent the variance around the mean date of each phenological event recorded for the species listed along the x-axis. Events are listed from l.h.s. to r.h.s. in the order in which they occurred on control plots, as in figure 1. Asterisks denote significant (p<0.05) differences among variances according to the F-test (see §2).
In our analysis of spatial variability of phenological events within plots, we detected a significant effect of year (F1,44=14.2, p<0.001) as well as of the warming treatment (F1,44=7.27, p=0.01), but the interaction between warming and year was not significant (F1,44=2.00, p=0.16). Averaged across both years, variability among all phenological events observed within plots was lower on treatment (mean±1 CV=18.4±1.76) than on control plots (25.1±1.22). The same pattern was apparent in 2003 (treatment mean=21.3±2.49; control mean=31.6±2.50) and 2004 (treatment mean=15.5±2.49; control mean=18.7±2.51) when means were estimated for each year individually. Hence, within plots, warming reduced the spatial variability in timing of events by approximately 27% for the pooled data, and by 33 and 17% in the first and second years, respectively, of the experiment. The difference between years may have been due to differences in ambient temperature and precipitation (Post et al. 2008). There were no significant random effects of plot (F=0.23, p=0.87) or site (F=0.04, p=0.97).
(d) Observed spatio-temporal variation in plant phenology and herbivore offspring production
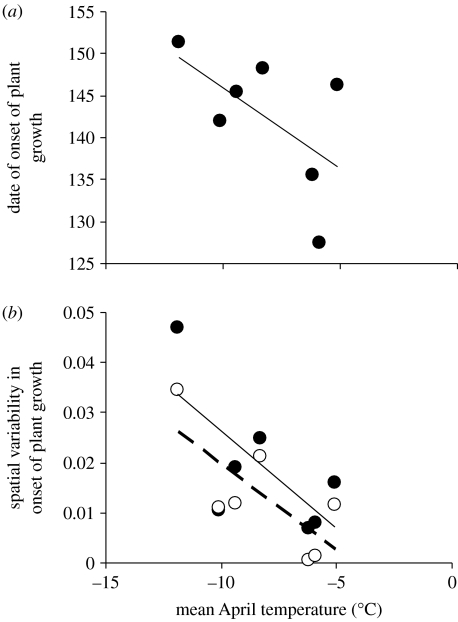
From 1993 to 2007, there was a trend towards earlier onset of the plant growing season in warmer years. Annual dates of 5% emergence of plant species on our observational plots displayed a negative association with mean April temperature (r=−0.59, p=0.17; figure 3a). Furthermore, just as our warming experiment revealed a spatial compression in the timing of plant phenological events within and among experimental plots in comparison with control plots, our observational data revealed a negative association between natural variation in springtime temperatures and spatial variability in progression of the plant growing season across the local landscape. Both of our indices of spatial variability in progression of plant growth were negatively correlated with mean April temperature (s.e.b across plots: r=−0.70, p=0.08; s.e.mean b among plots: r=−0.74, p=0.06; figure 3b). Hence, spring warming not only advanced the onset of plant growth but it also led to spatial compression of the plant growing season.
Figure 3.
Relationships between (a) the date of onset of the annual plant growing season, defined as the date at which 5% of plant species had emerged or (b) spatial variability in the date of onset of the plant growing season, defined as the standard error of the estimate of the date of onset of the growing season at our study site near Kangerlussuaq, West Greenland, and mean April temperature in 1993 and from 2002 to 2007. Temperature was recorded at Kangerlussuaq International Airport. See §2 for descriptions of estimation of the dependent variables in (a,b).
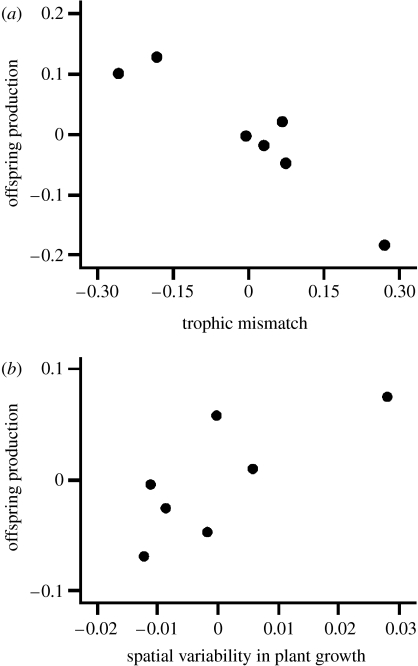
Our index of trophic mismatch and spatial variation in progression of the plant growing season combined explained 90% of the observed variation in offspring production by caribou among years (F2,4=17.8, R2=0.90, p=0.01). The overall best-fit model explaining interannual variation in offspring production included both predictor variables (AICc=−44.35, compared with −30.14 for the model with trophic mismatch only and −30.86 for the model with spatial variability in phenology only). Most of the variance in offspring production was explained by the index of trophic mismatch (partial r=−0.89, p=0.005; figure 4a), while less of the remaining variance was attributable to spatial variability in progression of the plant growing season (partial r=0.75, p=0.05; figure 4b). Despite the fact that a simple correlation between offspring production and spatial variability in the progression of the plant growing season was not significant (r=0.31, p=0.49), our regression model indicates that this factor explains residual variation in offspring production after accounting for trophic mismatch. Hence, when plant growth occurs later and more variably in space across the local landscape, offspring production by caribou is higher than it is in years when the timing and progression of plant growth is compressed spatially across the local landscape.
Figure 4.
Relationships between annual offspring production by caribou (proportion calves in the population of adult females) and (a) the degree of trophic mismatch each year and (b) the spatial variability in timing of plant growth each year at our study site near Kangerlussuaq, West Greenland, in 1993 and from 2002 to 2007. Both plots show partial correlations from the best-fit regression model. See §2 for details on estimation of the dependent and independent variables.
4. Discussion
Selective foraging by migratory herbivores reflects a strategy aimed at prolonging the intake of newly emergent, highly digestible and nutrient-rich plants (Senft et al. 1987). Selectivity in large ruminants occurs at multiple spatial scales, from the landscape and local scales (Mårell & Edenius 2006; Hebblewhite et al. 2008) to the forage patch (Mårell et al. 2002), and even to the scale of individual plants and plant parts (Bryant & Kuropat 1980; Trudell & White 1981). In far northern environments, this strategy is necessary for successful reproduction because resource availability is highly pulsed in time and heterogeneously distributed across the landscape and is viable precisely owing to the interaction between temporal and spatial dynamics of resource availability. Our results here and elsewhere (Post & Forchhammer 2008) emphasize that the mismatch not only in time but also in space, between peaks of resource availability and demand by reproducing individuals, are aspects of climate change that may have consequences for offspring production.
However, several cautionary notes are warranted. Our observations suggest that herbivore productivity was adversely associated with both the advancement of springtime availability of forage resources and the compression of resource availability spatially (figure 4). Nonetheless, we acknowledge that the short-term nature of both the observational, and more especially, experimental components of our study must be taken into consideration in interpretation of our results. Moreover, we recognize that the spatial scale of both our experiment and observations do not match completely the scale of the entire calving range occupied by this caribou population. Although offspring production by caribou in this population declined with reductions in spatial variability in plant phenology on their calving range, this relationship was significant only after accounting for the adverse effect of temporal advancement of plant phenology (figure 4). Moreover, we were previously unable (Post & Forchhammer 2008) to link conclusively the temporal component of trophic mismatch to offspring mortality in this population, so the extent to which further changes in either temporal or spatial aspects of plant phenological response to climatic warming will influence caribou population dynamics remains unaddressed, but worthy, in our estimation, of further consideration. Finally, although the patterns of temporal advancement and spatial compression of plant phenological events by warming revealed by our experiment were both corroborated by patterns of temporal and spatial variation in plant phenology in response to natural spring warming at the larger scale of our 7-year observational study (figure 3), neither of these relationships in the observational data were significant at the rigorous 0.05 level. Therefore, we cannot conclusively state that future warming at this site will further exacerbate spatial and temporal aspects of trophic mismatch for caribou, or influence their population dynamics, but we believe this area of research deserves further attention.
Several recent studies have focused on spatial patterns of interaction between climate change, plant phenology and herbivore population ecology. Invariably, however, those studies have used a regional landscape-scale approach, comparing patterns among populations of herbivores, and in this regard we believe our approach constitutes an important complement to such studies. Mysterud et al. (2001), for instance, reported in a comparison of harvested red deer among 105 municipalities spanning hundreds of kilometres in Norway that body weight correlated positively with landscape heterogeneity, namely altitudinal variation. Mysterud et al. (2001) concluded that this relationship reflected interactions between landscape heterogeneity, abiotic conditions and spatial patterns of plant phenology, but did not include actual measures of plant phenology in their study. By contrast, Herfindal et al. (2006), despite having conducted a similar comparison of variation in moose body weights among seven regions spanning the same spatial scale in Norway, documented a negative relationship between altitude and body weights. Herfindal et al. (2006), however, incorporated an NDVI of plant phenology in their analysis and found a positive relationship between body weights and a composite measure of plant phenology.
In contrast to such studies, ours, focused on spatial dynamics of plant phenology at local and intermediate spatial scales, though admittedly not at the same scale as the entire calving range of the focal population. This study was explicitly designed to experimentally test relationships between warming and spatial dynamics of plant phenology documented in previous large-scale analyses (Post & Stenseth 1999; Pettorelli et al. 2005a), and to determine whether those patterns were reflected at smaller spatial scales relevant to the foraging horizon of individual large herbivores. Our results indicate they were not.
In a broader ecological context, the spatial compression of plant phenology by experimental and observed warming documented here might represent a heretofore overlooked aspect of trophic mismatch induced by climate change. Most studies of the consequences of trophic mismatch for reproduction and population dynamics have focused on birds, where the emphasis has been on the temporal mismatch between resource availability and resource demand (Both et al. 2004). In migratory herbivores, however, arrival at the breeding grounds is not the end of the story. Herbivores track phenological progression of forage plants by moving across the local landscape to maintain intake of newly emergent species, individual plants or even tissues within plants (Klein 1970, 1990; Skogland 1989; Hebblewhite et al. 2008). Such observations suggest that any spatial constriction of the plant growing season might constrain the ability of migratory herbivores to prolong intake of high-quality forage during peak resource demand. Furthermore, such a constraint might negatively affect physical condition (Mysterud et al. 2001) and offspring production and survival (Post & Forchhammer 2008).
Our results may have general significance to herbivores inhabiting seasonal environments. Large herbivores prefer newly emergent forage, presumably owing to the high digestibility and nutrient content of young plant tissues (Hebblewhite et al. 2008). Plants consumed by reindeer (R. tarandus) on tundra, for instance, can be as much as 10% more digestible than the average available (White & Trudell 1980). In a famous example of multiplier effects in ecology, White (1983) documented that selective foraging by female reindeer resulted in a 27% increase in dry matter intake, a 45% increase in digestible dry matter intake, a 45% increase in metabolizable energy intake, a 267% increase in net energy for production and a 268% increase in body weight gain over non-selective foraging. Such selectivity occurred within a foraging radius of 10 m (White & Trudell 1980; White 1983), relatively the same scale, ecologically, as that at which our experiment was conducted. Our experiment revealed that warming reduced the spatial variability of plant phenology at the plot scale (1.5 m or less) by approximately 27% and at the local scale (hundreds of metres) by over twice that amount. Furthermore, our multi-annual observations revealed an approximately 80% reduction in local-scale variability in plant phenology between the coldest and warmest years (figure 3b). Reductions of that magnitude associated with future warming could conceivably impair the ability of herbivores such as caribou to forage selectively, with adverse consequences for their productivity. We suggest, therefore, that it is highly relevant to herbivore ecology to consider the manner in which warming will alter spatial patterns of plant phenology at more immediate spatial scales than that of the regional landscape.
Acknowledgments
Procedures for observation of animals were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University.
This research was conceived of by E.P. and supported by grants to him from the University of Alaska-Fairbanks, the U.S. National Science Foundation and The Committee for Research and Exploration of the National Geographic Society. For invaluable assistance with fieldwork, we thank Tom Adams, Pernille Bøving, Toke T. Høye, Syrena Johnson, Megan MacArthur, Ieva Perkons, Taylor Rees and Tyler Yenter. For logistical support, we thank the staff of VECO Polar Resources and the staff of Kangerlussuaq International Science Support. We thank Prof. N. C. Stenseth and four anonymous referees for their constructive comments on a previous manuscript.
References
- Albon S.D, Langvatn R. Plant phenology and the benefits of migration in a temperate ungulate. Oikos. 1992;65:502–513. doi:10.2307/3545568 [Google Scholar]
- Both C, Visser M.E. The effect of climate change on the correlation between avian life-history traits. Glob. Change Biol. 2005;11:1606–1613. doi:10.1111/j.1365-2486.2005.01038.x [Google Scholar]
- Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B. 2004;271:1657–1662. doi: 10.1098/rspb.2004.2770. doi:10.1098/rspb.2004.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J.P, Kuropat P.J. Selection of winter forage by sub-arctic browsing vertebrates—the role of plant chemistry. Annu. Rev. Ecol. Syst. 1980;11:261–285. doi:10.1146/annurev.es.11.110180.001401 [Google Scholar]
- Chen X.Q, Hu B, Yu R. Spatial and temporal variation of phenological growing season and climate change impacts in temperate eastern China. Glob. Change Biol. 2005;11:1118–1130. doi:10.1111/j.1365-2486.2005.00974.x [Google Scholar]
- Collins G.H, Hinkes M.T, Aderman A.R, Woolington J.D. Population growth, movements, and status of the Nushagak Peninsula caribou herd following reintroduction, 1988–2000. Rangifer. 2003;14:143–151. [Google Scholar]
- Ferguson S.H, Elkie P.C. Seasonal movement patterns of woodland caribou (Rangifer tarandus caribou) J. Zool. 2004;262:125–134. doi:10.1017/S0952836903004552 [Google Scholar]
- Ferguson S.H, Mahoney S.P. The relationship between weather and caribou productivity for the La-Poile caribou herd, Newfoundland. Rangifer. 1991;7:151–156. [Google Scholar]
- Giesel J.T. Reproductive strategies as adaptations to life in temporally heterogeneous environments. Annu. Rev. Ecol. Syst. 1976;7:57–79. doi:10.1146/annurev.es.07.110176.000421 [Google Scholar]
- Gunn A, Skogland T. Responses of caribou and reindeer to global warming. In: Oechel W.C, Callaghan T, Gilmanov T, Holten J.I, Maxwell B, Molau U, Sveinbjörnsson B, editors. Global change and Arctic terrestrial ecosystems. Springer; New York, NY: 1997. pp. 189–200. [Google Scholar]
- Haskell S.P, Ballard W.B. Factors limiting productivity of the Central Arctic caribou herd of Alaska. Rangifer. 2004;24:71–78. [Google Scholar]
- Hebblewhite M, Merrill E, McDermid G. A multi-scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecol. Monogr. 2008;78:141–166. [Google Scholar]
- Herfindal I, Solberg E.J, Sæther B.-E, Høgda K.A, Andersen R. Environmental phenology and geographical gradients in moose body mass. Oecologia. 2006;150:213–224. doi: 10.1007/s00442-006-0519-8. doi:10.1007/s00442-006-0519-8 [DOI] [PubMed] [Google Scholar]
- Kingsolver J.G, Pfennig D.W, Servedio M.R. Migration, local adaptation and the evolution of plasticity. Trends Ecol. Evol. 2002;17:540–541. doi:10.1016/S0169-5347(02)02641-1 [Google Scholar]
- Klein D.R. Introduction, increase, and crash of reindeer on St. Matthew Island. J. Wildl. Manage. 1968;32:350–367. doi:10.2307/3798981 [Google Scholar]
- Klein D.R. Tundra ranges north of the boreal forest. J. Range Manage. 1970;23:8–14. doi:10.2307/3896000 [Google Scholar]
- Klein, D. R. 1990 Variation in quality of caribou and reindeer forage plants associated with season, plant part, and phenology. Rangifer (Special Issue) 3, 123–130.
- Larter N.C, Nagy J.A. Seasonal changes in the composition of the diets of Peary caribou and muskoxen on Banks Island. Polar Res. 2004;23:131–140. doi:10.1111/j.1751-8369.2004.tb00003.x [Google Scholar]
- Levin S.A. Population dynamics models in heterogeneous environments. Annu. Rev. Ecol. Syst. 1976;7:287–310. doi:10.1146/annurev.es.07.110176.001443 [Google Scholar]
- Loe L.E, et al. Climate predictability and breeding phenology in red deer: timing and synchrony of rutting and calving in Norway and France. J. Anim. Ecol. 2005;74:579–588. doi:10.1111/j.1365-2656.2005.00987.x [Google Scholar]
- Lundberg P. The evolution of partial migration in birds. Trends Ecol. Evol. 1988;3:172–175. doi: 10.1016/0169-5347(88)90035-3. doi:10.1016/0169-5347(88)90035-3 [DOI] [PubMed] [Google Scholar]
- Mårell A, Edenius L. Spatial heterogeneity and hierarchical feeding habitat selection by reindeer. Arct. Antarct. Alp. Res. 2006;38:413–420. doi:10.1657/1523-0430(2006)38[413:SHAHFH]2.0.CO;2 [Google Scholar]
- Mårell A, Ball J.P, Hofgaard A. Foraging and movement paths of female reindeer: insights from fractal analysis, correlated random walks, and Levy flights. Can. J. Zool. 2002;80:854–865. doi:10.1139/z02-061 [Google Scholar]
- Marion G.M, et al. Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob. Change Biol. 1997;3:20–32. doi:10.1111/j.1365-2486.1997.gcb136.x [Google Scholar]
- Mysterud A, Langvatn R, Yoccoz N.G, Stenseth N.C. Plant phenology, migration and geographical variation in body weight of a large herbivore: the effect of a variable topography. J. Anim. Ecol. 2001;70:915–923. doi:10.1046/j.0021-8790.2001.00559.x [Google Scholar]
- Pettorelli N, Mysterud A, Yoccoz N.G, Langvatn R, Stenseth N.C. Importance of climatological downscaling and plant phenology for red deer in heterogeneous landscapes. Proc. R. Soc. B. 2005a;272:2357–2364. doi: 10.1098/rspb.2005.3218. doi:10.1098/rspb.2005.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorelli N, Weladji R.B, Holand O, Mysterud A, Breie H, Stenseth N.C. The relative role of winter and spring conditions: linking climate and landscape-scale plant phenology to alpine reindeer body mass. Biol. Lett. 2005b;1:24–26. doi: 10.1098/rsbl.2004.0262. doi:10.1098/rsbl.2004.0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E. Large-scale climate synchronizes the timing of flowering by multiple species. Ecology. 2003;84:277–281. doi:10.1890/0012-9658(2003)084[0277:LSCSTT]2.0.CO;2 [Google Scholar]
- Post E, Forchhammer M.C. Climate change reduces reproductive success of an arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B. 2008;363:2369–2375. doi: 10.1098/rstb.2007.2207. doi:10.1098/rstb.2007.2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E, Klein D.R. Caribou calf production and seasonal range quality during a population decline. J. Wildl. Manage. 1999;63:335–345. doi:10.2307/3802517 [Google Scholar]
- Post E, Stenseth N.C. Climatic variability, plant phenology, and northern ungulates. Ecology. 1999;80:1322–1339. doi:10.1890/0012-9658(1999)080[1322:CVPPAN]2.0.CO;2 [Google Scholar]
- Post E, Bøving P.S, Pedersen C, MacArthur M.A. Synchrony between caribou calving and plant phenology in depredated and non-depredated populations. Can. J. Zool. 2003;81:1709–1714. doi:10.1139/z03-172 [Google Scholar]
- Post E, Pedersen C, Wilmers C.C, Forchhammer M.C. Phenological sequences reveal aggregate life history response to climatic warming. Ecology. 2008;89:363–370. doi: 10.1890/06-2138.1. doi:10.1890/06-2138.1 [DOI] [PubMed] [Google Scholar]
- Roughgarden J. Population dynamics in a spatially varying environment—how population size tracks spatial variation in carrying capacity. Am. Nat. 1974;108:649–664. doi:10.1086/282941 [Google Scholar]
- Sakamoto Y, Ishiguro M, Kitagawa G. K. T. K. Scientific Publishers; Tokyo, Japan: 1986. Akaike information criterion statistic. [Google Scholar]
- Senft R.L, Coughenour M.B, Bailey D.W, Rittenhouse L.R, Sala O.E, Swift D.M. Large herbivore foraging and ecological hierarchies. Bioscience. 1987;37:789–799. doi:10.2307/1310545 [Google Scholar]
- Skogland T. Comparative social organization of wild reindeer in relation to food, mates, and predator avoidance. Adv. Ethol. 1989;29:1–74. [Google Scholar]
- Trudell J, White R.G. The effect of forage structure and availability on food intake, biting rate, bite size and daily eating time of reindeer. J. Appl. Ecol. 1981;18:63–81. doi:10.2307/2402479 [Google Scholar]
- Visser M.E, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. doi:10.1098/rspb.2005.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E, van Noordwijk A.J, Tinbergen J.M, Lessells C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc. R. Soc. B. 1998;265:1867–1870. doi:10.1098/rspb.1998.0514 [Google Scholar]
- Visser, M. E., Both, C. & Lambrechts, M. M. 2004 Global climate change leads to mistimed avian reproduction. In Birds and climate change, vol. 35, pp. 89–110. London, UK: Academic Press Ltd.
- White R.G. Foraging patterns and their multiplier effects on productivity of northern ungulates. Oikos. 1983;40:377–384. doi:10.2307/3544310 [Google Scholar]
- White R.G, Trudell J. Habitat preference and forage consumption by reindeer and caribou near Atkasook, Alaska. Arct. Alp. Res. 1980;12:511–529. doi:10.2307/1550498 [Google Scholar]
- Wiens J.A. Population responses to patchy environments. Annu. Rev. Ecol. Syst. 1976;7:81–120. doi:10.1146/annurev.es.07.110176.000501 [Google Scholar]